Androgen Modulates Functions of Endothelial Progenitor Cells through Activated Egr1 Signaling
- PMID: 26697079
- PMCID: PMC4677258
- DOI: 10.1155/2016/7057894
Androgen Modulates Functions of Endothelial Progenitor Cells through Activated Egr1 Signaling
Abstract
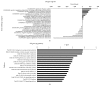
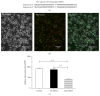
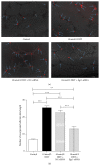
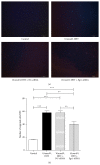
Researches show that androgens have important effects on migration of endothelial cells and endothelial protection in coronary heart disease. Endothelial progenitor cells (EPCs) as a progenitor cell type that can differentiate into endothelial cells, have a critical role in angiogenesis and endothelial protection. The relationship between androgen and the functions of EPCs has animated much interest and controversy. In this study, we investigated the angiogenic and migratory functions of EPCs after treatment by dihydrotestosterone (DHT) and the molecular mechanisms as well. We found that DHT treatment enhanced the incorporation of EPCs into tubular structures formed by HUVECs and the migratory activity of EPCs in the transwell assay dose dependently. Moreover, microarray analysis was performed to explore how DHT changes the gene expression profiles of EPCs. We found 346 differentially expressed genes in androgen-treated EPCs. Angiogenesis-related genes like Egr-1, Vcan, Efnb2, and Cdk2ap1 were identified to be regulated upon DHT treatment. Furthermore, the enhanced angiogenic and migratory abilities of EPCs after DHT treatment were inhibited by Egr1-siRNA transfection. In conclusion, our findings suggest that DHT markedly enhances the vessel forming ability and migration capacity of EPCs. Egr1 signaling may be a possible pathway in this process.
Figures


Similar articles
-
Dihydrotestosterone Augments the Angiogenic and Migratory Potential of Human Endothelial Progenitor Cells by an Androgen Receptor-Dependent Mechanism.Int J Mol Sci. 2024 Apr 29;25(9):4862. doi: 10.3390/ijms25094862. Int J Mol Sci. 2024. PMID: 38732080 Free PMC article.
-
Dihydrotestosterone modulates endothelial progenitor cell function via RhoA/ROCK pathway.Am J Transl Res. 2016 Oct 15;8(10):4300-4309. eCollection 2016. Am J Transl Res. 2016. PMID: 27830013 Free PMC article.
-
Androgens modulate male-derived endothelial cell homeostasis using androgen receptor-dependent and receptor-independent mechanisms.Angiogenesis. 2017 Feb;20(1):25-38. doi: 10.1007/s10456-016-9525-6. Epub 2016 Sep 27. Angiogenesis. 2017. PMID: 27679502
-
Endothelial progenitor cells in angiogenesis.Sheng Li Xue Bao. 2005 Feb 25;57(1):1-6. Sheng Li Xue Bao. 2005. PMID: 15719128 Review.
-
Dysfunction and Therapeutic Potential of Endothelial Progenitor Cells in Diabetes Mellitus.J Clin Med Res. 2018 Oct;10(10):752-757. doi: 10.14740/jocmr3581w. Epub 2018 Sep 10. J Clin Med Res. 2018. PMID: 30214646 Free PMC article. Review.
Cited by
-
Platelet‑derived growth factor D promotes the angiogenic capacity of endothelial progenitor cells.Mol Med Rep. 2019 Jan;19(1):125-132. doi: 10.3892/mmr.2018.9692. Epub 2018 Nov 26. Mol Med Rep. 2019. PMID: 30483778 Free PMC article.
-
Early Growth Response-1, an Integrative Sensor in Cardiovascular and Inflammatory Disease.J Am Heart Assoc. 2021 Nov 16;10(22):e023539. doi: 10.1161/JAHA.121.023539. Epub 2021 Nov 10. J Am Heart Assoc. 2021. PMID: 34755520 Free PMC article. Review.
-
Deciphering mechanisms of cardiomyocytes and non-cardiomyocyte transformation in myocardial remodeling of permanent atrial fibrillation.J Adv Res. 2024 Jul;61:101-117. doi: 10.1016/j.jare.2023.09.012. Epub 2023 Sep 16. J Adv Res. 2024. PMID: 37722560 Free PMC article.
-
Endothelial progenitor cells overexpressing platelet derived growth factor-D facilitate deep vein thrombosis resolution.J Thromb Thrombolysis. 2022 Apr;53(3):750-760. doi: 10.1007/s11239-021-02567-4. Epub 2021 Oct 20. J Thromb Thrombolysis. 2022. PMID: 34669127
-
Endothelial progenitor cell-derived exosomes, loaded with miR-126, promoted deep vein thrombosis resolution and recanalization.Stem Cell Res Ther. 2018 Aug 23;9(1):223. doi: 10.1186/s13287-018-0952-8. Stem Cell Res Ther. 2018. Retraction in: Stem Cell Res Ther. 2019 Jun 11;10(1):162. doi: 10.1186/s13287-019-1264-3. PMID: 30139377 Free PMC article. Retracted.
References
-
- Dubey R. K., Jackson E. K. Estrogen-induced cardiorenal protection: potential cellular, biochemical, and molecular mechanisms. The American Journal of Physiology—Renal Physiology. 2001;280(3):F365–F388. - PubMed
-
- Martín-Merino E., Johansson S., Morris T., Rodríguez L. A. G. Androgen deprivation therapy and the risk of coronary heart disease and heart failure in patients with prostate cancer: a nested case-control study in UK primary care. Drug Safety. 2011;34(11):1061–1077. doi: 10.2165/11594540-000000000-00000. - DOI - PubMed
-
- Ng M. K. C., Quinn C. M., McCrohon J. A., et al. Androgens up-regulate atherosclerosis-related genes in macrophages from males but not females: molecular insights into gender differences in atherosclerosis. Journal of the American College of Cardiology. 2003;42(7):1306–1313. doi: 10.1016/j.jacc.2003.07.002. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

