Dose-dependent, therapeutic potential of angiotensin-(1-7) for the treatment of pulmonary arterial hypertension
- PMID: 26697172
- PMCID: PMC4671739
- DOI: 10.1086/683696
Dose-dependent, therapeutic potential of angiotensin-(1-7) for the treatment of pulmonary arterial hypertension
Abstract
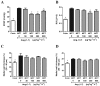
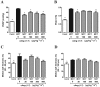
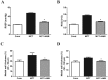
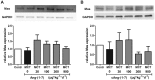
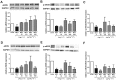
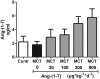
The effects of the heptapeptide angiotensin-(1-7) (Ang-(1-7)), via its receptor Mas, oppose many of the effects of the classic angiotensin II signaling pathway, and pharmacological exploitation of this effect is currently actively pursued for a wide range of cardiovascular, neoplastic, or immunological disorders. On the basis of its vasodilatory and antiproliferative properties, Ang-(1-7) has consequentially also been proposed as a novel therapeutic strategy for the treatment of pulmonary arterial hypertension (PAH). In this study, we tested the effectiveness of Ang-(1-7) and its stable, cyclic analog cAng-(1-7) over a range of doses for their therapeutic potential in experimental PAH. In the monocrotaline (MCT) rat model of PAH, Ang-(1-7) or cAng-(1-7) were injected in doses of 30, 100, 300, or 900 μg kg(-1) day(-1), and effects on pulmonary hemodynamics and vascular remodeling were assessed. Five weeks after MCT injection, right ventricular systolic pressure (RVSP) was significantly reduced for 3 dose groups treated with Ang-(1-7) (100, 300, and 900 μg kg(-1) day(-1)) and for all dose groups treated with cAng-(1-7), as compared to untreated controls, yet the total reduction of RVSP was <50% at best and thus markedly lower than that with a positive treatment control with ambrisentan. Medial-wall thickness in pulmonary arterioles was only slightly reduced, without reaching significance, for any of the tested Ang-(1-7) compounds and doses. The reported moderate attenuation of PAH does not confirm the previously postulated high promise of this strategy, and the therapeutic usefulness of Ang-(1-7) may be limited in PAH relative to that in other cardiovascular diseases.
Keywords: pulmonary hypertension; renin-angiotensin system; vascular remodeling.
Figures
Similar articles
-
Ang-(1-7) might prevent the development of monocrotaline induced pulmonary arterial hypertension in rats.Eur Rev Med Pharmacol Sci. 2011 Jan;15(1):1-7. Eur Rev Med Pharmacol Sci. 2011. PMID: 21381494
-
Mesenchymal stem cell-derived microvesicles alleviate pulmonary arterial hypertension by regulating renin-angiotensin system.J Am Soc Hypertens. 2018 Jun;12(6):470-478. doi: 10.1016/j.jash.2018.02.006. Epub 2018 Mar 15. J Am Soc Hypertens. 2018. PMID: 29752040
-
Angiotensin-(1-9) ameliorates pulmonary arterial hypertension via angiotensin type II receptor.Korean J Physiol Pharmacol. 2018 Jul;22(4):447-456. doi: 10.4196/kjpp.2018.22.4.447. Epub 2018 Jun 25. Korean J Physiol Pharmacol. 2018. PMID: 29962859 Free PMC article.
-
A urotensin II receptor antagonist, KR36676, decreases vascular remodeling and inflammation in experimental pulmonary hypertension.Int Immunopharmacol. 2016 Nov;40:196-202. doi: 10.1016/j.intimp.2016.09.002. Epub 2016 Sep 6. Int Immunopharmacol. 2016. PMID: 27611861
-
The vasoprotective axes of the renin-angiotensin system: Physiological relevance and therapeutic implications in cardiovascular, hypertensive and kidney diseases.Pharmacol Res. 2017 Nov;125(Pt A):21-38. doi: 10.1016/j.phrs.2017.06.005. Epub 2017 Jun 12. Pharmacol Res. 2017. PMID: 28619367 Free PMC article. Review.
Cited by
-
Synergism between Angiotensin receptors ligands: Role of Angiotensin-(1-7) in modulating AT2 R agonist response on nitric oxide in kidney cells.Pharmacol Res Perspect. 2020 Dec;8(6):e00667. doi: 10.1002/prp2.667. Pharmacol Res Perspect. 2020. PMID: 33197136 Free PMC article.
-
Targeting Neprilysin (NEP) pathways: A potential new hope to defeat COVID-19 ghost.Biochem Pharmacol. 2020 Aug;178:114057. doi: 10.1016/j.bcp.2020.114057. Epub 2020 May 27. Biochem Pharmacol. 2020. PMID: 32470547 Free PMC article. Review.
-
Neurohormonal modulation in pulmonary arterial hypertension.Eur Respir J. 2021 Oct 28;58(4):2004633. doi: 10.1183/13993003.04633-2020. Print 2021 Oct. Eur Respir J. 2021. PMID: 33766951 Free PMC article. Review.
-
Metformin prevents the development of monocrotaline-induced pulmonary hypertension by decreasing serum levels of big endothelin-1.Exp Ther Med. 2020 Dec;20(6):149. doi: 10.3892/etm.2020.9278. Epub 2020 Oct 6. Exp Ther Med. 2020. PMID: 33093887 Free PMC article.
-
Pathophysiology and potential future therapeutic targets using preclinical models of COVID-19.ERJ Open Res. 2020 Dec 7;6(4):00405-2020. doi: 10.1183/23120541.00405-2020. eCollection 2020 Oct. ERJ Open Res. 2020. PMID: 33313306 Free PMC article. Review.
References
-
- Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2009;54(1 suppl.):S43–S54. - PubMed
-
- Galiè N, Manes A, Branzi A. The endothelin system in pulmonary arterial hypertension. Cardiovasc Res 2004;61(2):227–337. - PubMed
-
- Hampl V, Herget J. Role of nitric oxide in the pathogenesis of chronic pulmonary hypertension. Physiol Rev 2000;80(4):1337–1372. - PubMed
-
- Rhodes CJ, Davidson A, Gibbs JSR, Wharton J, Wilkins MR. Therapeutic targets in pulmonary arterial hypertension. Pharmacol Ther 2009;121(1):69–88. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

