Inhibition of ACE Retards Tau Hyperphosphorylation and Signs of Neuronal Degeneration in Aged Rats Subjected to Chronic Mild Stress
- PMID: 26697495
- PMCID: PMC4677170
- DOI: 10.1155/2015/917156
Inhibition of ACE Retards Tau Hyperphosphorylation and Signs of Neuronal Degeneration in Aged Rats Subjected to Chronic Mild Stress
Abstract
With increasing life expectancy, Alzheimer's disease (AD) and other types of age-associated dementia are on the rise worldwide. Treatment approaches for dementia are insufficient and novel therapies are not readily available. In this context repurposing of established drugs appears attractive. A well-established class of cardiovascular drugs, which targets the angiotensin II system, is such a candidate, which currently undergoes a paradigm shift with regard to the potential benefit for treatment of neurodegenerative symptoms. In search for additional evidence, we subjected aged rats to chronic unpredictable mild stress, which is known to enhance the development of AD-related neuropathological features. We report here that four weeks of chronic mild stress induced a strong upregulation of the hippocampal angiotensin-converting enzyme (Ace) at gene expression and protein level. Concomitantly, tau protein hyperphosphorylation developed. Signs of neurodegeneration were detected by the significant downregulation of neuronal structure proteins such as microtubule-associated protein 2 (Map2) and synuclein-gamma (Sncg). Ace was involved in neurodegenerative symptoms because treatment with the brain-penetrating ACE inhibitor, captopril, retarded tau hyperphosphorylation and signs of neurodegeneration. Moreover, ACE inhibitor treatment could counteract glutamate neurotoxicity by preventing the downregulation of glutamate decarboxylase 2 (Gad2). Taken together, ACE inhibition targets neurodegeneration triggered by environmental stress.
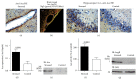
Figures



References
-
- Scully T. Demography: to the limit. Nature. 2012;492(7427):S2–S3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

