Corticosterone Blocks Ovarian Cyclicity and the LH Surge via Decreased Kisspeptin Neuron Activation in Female Mice
- PMID: 26697722
- PMCID: PMC4769373
- DOI: 10.1210/en.2015-1711
Corticosterone Blocks Ovarian Cyclicity and the LH Surge via Decreased Kisspeptin Neuron Activation in Female Mice
Abstract
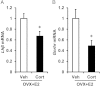
Stress elicits activation of the hypothalamic-pituitary-adrenal axis, which leads to enhanced circulating glucocorticoids, as well as impaired gonadotropin secretion and ovarian cyclicity. Here, we tested the hypothesis that elevated, stress-levels of glucocorticoids disrupt ovarian cyclicity by interfering with the preovulatory sequence of endocrine events necessary for the LH surge. Ovarian cyclicity was monitored in female mice implanted with a cholesterol or corticosterone (Cort) pellet. Cort, but not cholesterol, arrested cyclicity in diestrus. Subsequent studies focused on the mechanism whereby Cort stalled the preovulatory sequence by assessing responsiveness to the positive feedback estradiol signal. Ovariectomized mice were treated with an LH surge-inducing estradiol implant, as well as Cort or cholesterol, and assessed several days later for LH levels on the evening of the anticipated surge. All cholesterol females showed a clear LH surge. At the time of the anticipated surge, LH levels were undetectable in Cort-treated females. In situ hybridization analyses the anteroventral periventricular nucleus revealed that Cort robustly suppressed the percentage of Kiss1 cells coexpressing cfos, as well as reduced the number of Kiss1 cells and amount of Kiss1 mRNA per cell, compared with expression in control brains. In addition, Cort blunted pituitary expression of the genes encoding the GnRH receptor and LHβ, indicating inhibition of gonadotropes during the blockage of the LH surge. Collectively, our findings support the hypothesis that physiological stress-levels of Cort disrupts ovarian cyclicity, in part, through disruption of positive feedback mechanisms at both the hypothalamic and pituitary levels which are necessary for generation of the preovulatory LH surge.
Figures





Similar articles
-
Sex Differences in Steroid Receptor Coexpression and Circadian-Timed Activation of Kisspeptin and RFRP-3 Neurons May Contribute to the Sexually Dimorphic Basis of the LH Surge.Endocrinology. 2017 Oct 1;158(10):3565-3578. doi: 10.1210/en.2017-00405. Endocrinology. 2017. PMID: 28938464 Free PMC article.
-
Reduced responsiveness of kisspeptin neurons to estrogenic positive feedback associated with age-related disappearance of LH surge in middle-age female rats.Gen Comp Endocrinol. 2013 Nov 1;193:121-9. doi: 10.1016/j.ygcen.2013.06.024. Epub 2013 Jul 10. Gen Comp Endocrinol. 2013. PMID: 23851104
-
Significance of neonatal testicular sex steroids to defeminize anteroventral periventricular kisspeptin neurons and the GnRH/LH surge system in male rats.Biol Reprod. 2009 Dec;81(6):1216-25. doi: 10.1095/biolreprod.109.078311. Epub 2009 Aug 14. Biol Reprod. 2009. PMID: 19684332
-
Mechanisms for endotoxin-induced disruption of ovarian cyclicity: observations in sheep.Reprod Suppl. 2002;59:101-13. Reprod Suppl. 2002. PMID: 12698976 Review.
-
Metastin/kisspeptin and control of estrous cycle in rats.Rev Endocr Metab Disord. 2007 Mar;8(1):21-9. doi: 10.1007/s11154-007-9032-6. Rev Endocr Metab Disord. 2007. PMID: 17377846 Review.
Cited by
-
Complementary and Alternative Medicine for Premature Ovarian Insufficiency: A Review of Utilization and Mechanisms.Evid Based Complement Alternat Med. 2022 Apr 1;2022:9053930. doi: 10.1155/2022/9053930. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35399635 Free PMC article. Review.
-
Glucocorticoid therapy in assisted reproduction.Clin Exp Reprod Med. 2021 Dec;48(4):295-302. doi: 10.5653/cerm.2021.04819. Epub 2021 Nov 30. Clin Exp Reprod Med. 2021. PMID: 34875736 Free PMC article.
-
Acute Psychosocial Stress Inhibits LH Pulsatility and Kiss1 Neuronal Activation in Female Mice.Endocrinology. 2017 Nov 1;158(11):3716-3723. doi: 10.1210/en.2017-00301. Endocrinology. 2017. PMID: 28973125 Free PMC article.
-
The transcription factor VAX1 in VIP neurons of the suprachiasmatic nucleus impacts circadian rhythm generation, depressive-like behavior, and the reproductive axis in a sex-specific manner in mice.Front Endocrinol (Lausanne). 2023 Dec 22;14:1269672. doi: 10.3389/fendo.2023.1269672. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 38205198 Free PMC article.
-
Regulation of the gonadotropin-releasing hormone neuron during stress.J Neuroendocrinol. 2022 May;34(5):e13098. doi: 10.1111/jne.13098. Epub 2022 Feb 6. J Neuroendocrinol. 2022. PMID: 35128742 Free PMC article. Review.
References
-
- Li XF, Bowe JE, Kinsey-Jones JS, Brain SD, Lightman SL, O'Byrne KT. Differential role of corticotrophin-releasing factor receptor types 1 and 2 in stress-induced suppression of pulsatile luteinising hormone secretion in the female rat. J Neuroendocrinol. 2006;18:602–610. - PubMed
-
- Tilbrook AJ, Turner AI, Clarke IJ. Effects of stress on reproduction in non-rodent mammals: the role of glucocorticoids and sex differences. Rev Reprod. 2000;5:105–113. - PubMed
-
- Tilbrook AJ, Turner AI, Clarke IJ. Stress and reproduction: central mechanisms and sex differences in non-rodent species. Stress. 2002;5:83–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

