Sterilization of Lung Matrices by Supercritical Carbon Dioxide
- PMID: 26697757
- PMCID: PMC4782026
- DOI: 10.1089/ten.TEC.2015.0449
Sterilization of Lung Matrices by Supercritical Carbon Dioxide
Abstract
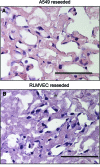
Lung engineering is a potential alternative to transplantation for patients with end-stage pulmonary failure. Two challenges critical to the successful development of an engineered lung developed from a decellularized scaffold include (i) the suppression of resident infectious bioburden in the lung matrix, and (ii) the ability to sterilize decellularized tissues while preserving the essential biological and mechanical features intact. To date, the majority of lungs are sterilized using high concentrations of peracetic acid (PAA) resulting in extracellular matrix (ECM) depletion. These mechanically altered tissues have little to no storage potential. In this study, we report a sterilizing technique using supercritical carbon dioxide (ScCO2) that can achieve a sterility assurance level 10(-6) in decellularized lung matrix. The effects of ScCO2 treatment on the histological, mechanical, and biochemical properties of the sterile decellularized lung were evaluated and compared with those of freshly decellularized lung matrix and with PAA-treated acellular lung. Exposure of the decellularized tissue to ScCO2 did not significantly alter tissue architecture, ECM content or organization (glycosaminoglycans, elastin, collagen, and laminin), observations of cell engraftment, or mechanical integrity of the tissue. Furthermore, these attributes of lung matrix did not change after 6 months in sterile buffer following sterilization with ScCO2, indicating that ScCO2 produces a matrix that is stable during storage. The current study's results indicate that ScCO2 can be used to sterilize acellular lung tissue while simultaneously preserving key biological components required for the function of the scaffold for regenerative medicine purposes.
Figures





Similar articles
-
Decellularized heart ECM hydrogel using supercritical carbon dioxide for improved angiogenesis.Acta Biomater. 2018 Feb;67:270-281. doi: 10.1016/j.actbio.2017.11.046. Epub 2017 Dec 6. Acta Biomater. 2018. PMID: 29223704
-
Development of a Sterile Amniotic Membrane Tissue Graft Using Supercritical Carbon Dioxide.Tissue Eng Part C Methods. 2015 Jul;21(7):649-59. doi: 10.1089/ten.TEC.2014.0304. Epub 2015 Mar 6. Tissue Eng Part C Methods. 2015. PMID: 25471248
-
Scaffold-based tissue engineering: Supercritical carbon dioxide as an alternative method for decellularization and sterilization of dense materials.Acta Biomater. 2023 Jan 1;155:323-332. doi: 10.1016/j.actbio.2022.11.028. Epub 2022 Nov 22. Acta Biomater. 2023. PMID: 36423818
-
Development of decellularized scaffolds for stem cell-driven tissue engineering.J Tissue Eng Regen Med. 2017 Apr;11(4):942-965. doi: 10.1002/term.2061. Epub 2015 Jun 29. J Tissue Eng Regen Med. 2017. PMID: 26119160 Review.
-
Supercritical CO2 technology: The next standard sterilization technique?Mater Sci Eng C Mater Biol Appl. 2019 Jun;99:520-540. doi: 10.1016/j.msec.2019.01.121. Epub 2019 Jan 31. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 30889727 Review.
Cited by
-
Preparation and Use of Decellularized Extracellular Matrix for Tissue Engineering.J Funct Biomater. 2022 Nov 14;13(4):240. doi: 10.3390/jfb13040240. J Funct Biomater. 2022. PMID: 36412881 Free PMC article. Review.
-
Humidified Warmed CO2 Treatment Therapy Strategies Can Save Lives With Mitigation and Suppression of SARS-CoV-2 Infection: An Evidence Review.Front Med (Lausanne). 2020 Dec 11;7:594295. doi: 10.3389/fmed.2020.594295. eCollection 2020. Front Med (Lausanne). 2020. PMID: 33425942 Free PMC article.
-
Recent advances in soluble decellularized extracellular matrix for heart tissue engineering and organ modeling.J Biomater Appl. 2023 Nov;38(5):577-604. doi: 10.1177/08853282231207216. J Biomater Appl. 2023. PMID: 38006224 Free PMC article. Review.
-
Decellularized vascularized bone grafts: A preliminary in vitro porcine model for bioengineered transplantable bone shafts.Front Bioeng Biotechnol. 2023 Jan 18;10:1003861. doi: 10.3389/fbioe.2022.1003861. eCollection 2022. Front Bioeng Biotechnol. 2023. PMID: 36743653 Free PMC article.
-
Emerging Paradigms in Bioengineering the Lungs.Bioengineering (Basel). 2022 May 1;9(5):195. doi: 10.3390/bioengineering9050195. Bioengineering (Basel). 2022. PMID: 35621473 Free PMC article. Review.
References
-
- Gilpin S.E., and Ott H.C. Using nature's platform to engineer bio-artificial lungs. Ann Am Thorac Soc 12 Suppl 1, S45, 2015 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

