The Physiology of Adventitious Roots
- PMID: 26697895
- PMCID: PMC4734560
- DOI: 10.1104/pp.15.01360
The Physiology of Adventitious Roots
Abstract
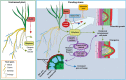
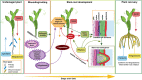
Adventitious roots are plant roots that form from any nonroot tissue and are produced both during normal development (crown roots on cereals and nodal roots on strawberry [Fragaria spp.]) and in response to stress conditions, such as flooding, nutrient deprivation, and wounding. They are important economically (for cuttings and food production), ecologically (environmental stress response), and for human existence (food production). To improve sustainable food production under environmentally extreme conditions, it is important to understand the adventitious root development of crops both in normal and stressed conditions. Therefore, understanding the regulation and physiology of adventitious root formation is critical for breeding programs. Recent work shows that different adventitious root types are regulated differently, and here, we propose clear definitions of these classes. We use three case studies to summarize the physiology of adventitious root development in response to flooding (case study 1), nutrient deficiency (case study 2), and wounding (case study 3).
© 2016 American Society of Plant Biologists. All Rights Reserved.
Figures


References
-
- Ahkami AH, Lischewski S, Haensch KT, Porfirova S, Hofmann J, Rolletschek H, Melzer M, Franken P, Hause B, Druege U, et al. (2009) Molecular physiology of adventitious root formation in Petunia hybrida cuttings: involvement of wound response and primary metabolism. New Phytol 181: 613–625 - PubMed
-
- Ahkami AH, Melzer M, Ghaffari MR, Pollmann S, Ghorbani Javid M, Shahinnia F, Hajirezaei MR, Druege U (2013) Distribution of indole-3-acetic acid in Petunia hybrida shoot tip cuttings and relationship between auxin transport, carbohydrate metabolism and adventitious root formation. Planta 238: 499–517 - PMC - PubMed
-
- Argus RE, Colmer TD, Grierson PF (2015) Early physiological flood tolerance is followed by slow post-flooding root recovery in the dryland riparian tree Eucalyptus camaldulensis subsp. refulgens. Plant Cell Environ 38: 1189–1199 - PubMed
-
- Armstrong W. (1971) Oxygen diffusion from the roots of rice grown under non-waterlogged conditions. Physiol Plant 24: 242–247
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

