Assessment of immune responses to H5N1 inactivated influenza vaccine among individuals previously primed with H5N2 live attenuated influenza vaccine
- PMID: 26697973
- PMCID: PMC5054797
- DOI: 10.1080/21645515.2015.1069931
Assessment of immune responses to H5N1 inactivated influenza vaccine among individuals previously primed with H5N2 live attenuated influenza vaccine
Abstract
During the past decade, a number of H5 subtype influenza vaccines have been developed and tested in clinical trials, but most of them induced poor serum antibody responses prompting the evaluation of novel vaccination approaches. One of the most promising ones is a "prime-boost" strategy, which could result in the induction of prompt and robust immune responses to a booster influenza vaccine following priming with homologous or heterologous vaccine strains. In our study we evaluated immunogenicity of an adjuvanted A(H5N1) inactivated influenza vaccine (IIV) in healthy adult subjects who received A(H5N2) live attenuated influenza vaccine (LAIV) 1.5 years earlier and compared this with a group of naïve subjects. We found that priming with A(H5N2) LAIV induced a long-lasting B-cell immunological memory against influenza A(H5N1) virus, which was brought on by more prompt and vigorous antibody production to a single dose of A(H5N1) IIV in the primed group, compared to the naïve controls. Thus, by day 28 after the first booster dose, the hemagglutination inhibition and neutralizing (MN) antibody titer rises were 17.2 and 30.8 in the primed group, compared to 2.3 and 8.0 in the control group, respectively. The majority (79%) of the primed individuals achieved seroprotective MN antibody titers at 7 days after the first dose of the IIV. All LAIV-primed volunteers had MN titers ≥ 1:40 by Day 28 after one dose of IIV, whereas only 58% subjects from the naïve control group developed similar immune responses at this time point. The second A(H5N1) IIV dose did not increase the immune response in the LAIV-primed group, whereas 2 doses of IIV were required for naïve volunteers to develop significant immune responses. These findings were of special significance since Russian-based LAIV technology has been licensed to WHO, through whom the vaccine has been provided to vaccine manufacturers in India, China and Thailand - countries particularly vulnerable to a pandemic influenza. The results of our study will be useful to inform the development of vaccination strategies in these countries in the event of a pandemic.
Keywords: H5 avian influenza viruses; clinical trial; inactivated influenza vaccine; live attenuated influenza vaccine; memory immune response; prime-boost strategy; safety.
Figures
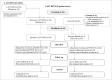


References
-
- WHO, WHO global influenza preparedness plan WHO/CDS/CSR/GIP/2005.5 http://www.who.int/csr/resources/publications/influenza/en/WHO_CDS_CSR_G.... 2005 - PubMed
-
- Anonymous, Development of a Clinical Trial Plan for Pandemic Influenza Vaccines Department of Health and Human Services. National Institute of Allergy and Infection Diseases; September 22–23, 2003, Bethesda, Maryland Meeting Summary. http://www.niaid.nih.gov/about/organization/dmid/Documents/pansummary.pd...
-
- WHO, Tables on clinical evaluation of influenza vaccines. http://www.who.int/immunization/diseases/influenza/clinical_evaluation_t.... WHO, Geneva, Switzerland: 2014.
-
- Rudenko L, Isakova-Sivak I. Pandemic Preparedness with Live Attenuated Influenza Vaccines Based on A/Leningrad/134/17/57 (H2N2) Master Donor Virus. Expert Review of Vaccines, 2015. 14(03): p:395-412; PMID:25555687; http://dx.doi.org/ 10.1586/14760584.2015.979159 - DOI - PubMed
-
- Coelingh KL, Luke CJ, Jin H, Talaat KR. Development of live attenuated influenza vaccines against pandemic influenza strains. Expert Rev Vaccines 2014. 13(7): p:855-71; PMID:24867587; http://dx.doi.org/ 10.1586/14760584.2014.922417 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
