Evaluation of mucosal adjuvants and immunization routes for the induction of systemic and mucosal humoral immune responses in macaques
- PMID: 26697975
- PMCID: PMC5054781
- DOI: 10.1080/21645515.2015.1070998
Evaluation of mucosal adjuvants and immunization routes for the induction of systemic and mucosal humoral immune responses in macaques
Abstract
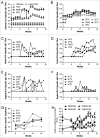
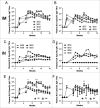
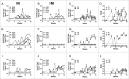
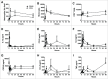
Delivering vaccine antigens to mucosal surfaces is potentially very attractive, especially as protection from mucosal infections may be mediated by local immune responses. However, to date mucosal immunization has had limited successes, with issues of both safety and poor immunogenicity. One approach to improve immunogenicity is to develop adjuvants that are effective and safe at mucosal surfaces. Differences in immune responses between mice and men have overstated the value of some experimental adjuvants which have subsequently performed poorly in the clinic. Due to their closer similarity, non-human primates can provide a more accurate picture of adjuvant performance. In this study we immunised rhesus macaques (Macaca mulatta) using a unique matrix experimental design that maximised the number of adjuvants screened while reducing the animal usage. Macaques were immunised by the intranasal, sublingual and intrarectal routes with the model protein antigens keyhole limpet haemocyanin (KLH), β-galactosidase (β-Gal) and ovalbumin (OVA) in combination with the experimental adjuvants Poly(I:C), Pam3CSK4, chitosan, Thymic Stromal Lymphopoietin (TSLP), MPLA and R848 (Resiquimod). Of the routes used, only intranasal immunization with KLH and R848 induced a detectable antibody response. When compared to intramuscular immunization, intranasal administration gave slightly lower levels of antigen specific antibody in the plasma, but enhanced local responses. Following intranasal delivery of R848, we observed a mildly inflammatory response, but no difference to the control. From this we conclude that R848 is able to boost antibody responses to mucosally delivered antigen, without causing excess local inflammation.
Keywords: Macaque; Mucosal; TLR ligand; adjuvant; inflammation; intranasal.
Figures
Similar articles
-
The ovine nasal mucosa: an alternative tissue site for mucosal immunization.Methods. 2006 Feb;38(2):112-6. doi: 10.1016/j.ymeth.2005.09.010. Methods. 2006. PMID: 16427306
-
Evaluation of mucosal and systemic immune responses elicited by GPI-0100- adjuvanted influenza vaccine delivered by different immunization strategies.PLoS One. 2013 Jul 31;8(7):e69649. doi: 10.1371/journal.pone.0069649. Print 2013. PLoS One. 2013. PMID: 23936066 Free PMC article.
-
Type I and Type III Interferons Differ in Their Adjuvant Activities for Influenza Vaccines.J Virol. 2019 Nov 13;93(23):e01262-19. doi: 10.1128/JVI.01262-19. Print 2019 Dec 1. J Virol. 2019. PMID: 31511392 Free PMC article.
-
Alternative routes of mucosal immunization in large animals.Immunol Cell Biol. 2004 Feb;82(1):10-6. doi: 10.1111/j.1440-1711.2004.01214.x. Immunol Cell Biol. 2004. PMID: 14984589 Review.
-
Dendritic cell-targeting DNA-based nasal adjuvants for protective mucosal immunity to Streptococcus pneumoniae.Microbiol Immunol. 2017 Jun;61(6):195-205. doi: 10.1111/1348-0421.12487. Microbiol Immunol. 2017. PMID: 28463465 Free PMC article. Review.
Cited by
-
Preventive HIV Vaccines-Leveraging on Lessons from the Past to Pave the Way Forward.Vaccines (Basel). 2021 Sep 8;9(9):1001. doi: 10.3390/vaccines9091001. Vaccines (Basel). 2021. PMID: 34579238 Free PMC article. Review.
-
SARS-CoV-2 sublingual vaccine with RBD antigen and poly(I:C) adjuvant: Preclinical study in cynomolgus macaques.Biol Methods Protoc. 2023 Sep 13;8(1):bpad017. doi: 10.1093/biomethods/bpad017. eCollection 2023. Biol Methods Protoc. 2023. PMID: 37711440 Free PMC article.
-
Intramuscular Immunisation with Chlamydial Proteins Induces Chlamydia trachomatis Specific Ocular Antibodies.PLoS One. 2015 Oct 26;10(10):e0141209. doi: 10.1371/journal.pone.0141209. eCollection 2015. PLoS One. 2015. PMID: 26501198 Free PMC article.
-
Formulation, inflammation, and RNA sensing impact the immunogenicity of self-amplifying RNA vaccines.Mol Ther Nucleic Acids. 2022 Dec 5;31:29-42. doi: 10.1016/j.omtn.2022.11.024. eCollection 2023 Mar 14. Mol Ther Nucleic Acids. 2022. PMID: 36589712 Free PMC article.
-
Highly Pathogenic Avian Influenza H5 Hemagglutinin Fused with the A Subunit of Type IIb Escherichia coli Heat Labile Enterotoxin Elicited Protective Immunity and Neutralization by Intranasal Immunization in Mouse and Chicken Models.Vaccines (Basel). 2019 Nov 22;7(4):193. doi: 10.3390/vaccines7040193. Vaccines (Basel). 2019. PMID: 31766677 Free PMC article.
References
-
- Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol 2012; 12:592-605; PMID:22828912; http://dx.doi.org/10.1038/nri3251 - DOI - PubMed
-
- Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol 2006; 6:148-58; PMID:16491139; http://dx.doi.org/10.1038/nri1777 - DOI - PubMed
-
- Mestecky J, McGhee JR, Michalek SM, Arnold RR, Crago SS, Babb JL. Concept of the local and common mucosal immune response. Adv Exp Med Biol 1978; 107:185-92 - PubMed
-
- Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med 2005; 11:S45-S53; PMID:15812489; http://dx.doi.org/10.1038/nm1213 - DOI - PubMed
-
- Newsted D, Fallahi F, Golshani A, Azizi A. Advances and challenges in mucosal adjuvant technology. Vaccine 2015; 33:2399-405; PMID:25865473; http://dx.doi.org/10.1016/j.vaccine.2015.03.096 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
