The Glt1 glutamate receptor mediates the establishment and perpetuation of chronic visceral pain in an animal model of stress-induced bladder hyperalgesia
- PMID: 26697981
- PMCID: PMC5504462
- DOI: 10.1152/ajprenal.00297.2015
The Glt1 glutamate receptor mediates the establishment and perpetuation of chronic visceral pain in an animal model of stress-induced bladder hyperalgesia
Abstract
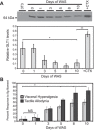
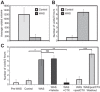
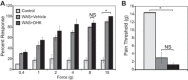
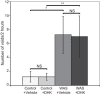
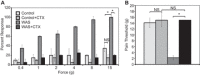
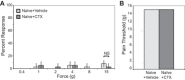
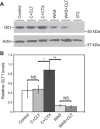
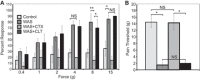
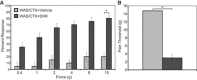
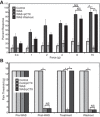
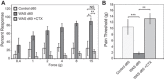
Psychological stress exacerbates interstitial cystitis/bladder pain syndrome (IC/BPS), a lower urinary tract pain disorder characterized by increased urinary frequency and bladder pain. Glutamate (Glu) is the primary excitatory neurotransmitter modulating nociceptive networks. Glt1, an astrocytic transporter responsible for Glu clearance, is critical in pain signaling termination. We sought to examine the role of Glt1 in stress-induced bladder hyperalgesia and urinary frequency. In a model of stress-induced bladder hyperalgesia with high construct validity to human IC/BPS, female Wistar-Kyoto (WKY) rats were subjected to 10-day water avoidance stress (WAS). Referred hyperalgesia and tactile allodynia were assessed after WAS with von Frey filaments. After behavioral testing, we assessed Glt1 expression in the spinal cord by immunoblotting. We also examined the influence of dihydrokainate (DHK) and ceftriaxone (CTX), which downregulate and upregulate Glt1, respectively, on pain development. Rats exposed to WAS demonstrated increased voiding frequency, increased colonic motility, anxiety-like behaviors, and enhanced visceral hyperalgesia and tactile allodynia. This behavioral phenotype correlated with decreases in spinal Glt1 expression. Exogenous Glt1 downregulation by DHK resulted in hyperalgesia similar to that following WAS. Exogenous Glt1 upregulation via intraperitoneal CTX injection inhibited the development of and reversed preexisting pain and voiding dysfunction induced by WAS. Repeated psychological stress results in voiding dysfunction and hyperalgesia that correlate with altered central nervous system glutamate processing. Manipulation of Glu handling altered the allodynia developing after psychological stress, implicating Glu neurotransmission in the pathophysiology of bladder hyperalgesia in the WAS model of IC/BPS.
Keywords: bladder pain syndrome; ceftriaxone; glutamate processing; hyperalgesia; interstitial cystitis.
Copyright © 2016 the American Physiological Society.
Figures
References
-
- Barsky AJ, Borus JF. Functional somatic syndromes. Ann Intern Med 130: 910–921, 1999. - PubMed
-
- Berry JD, Shefner JM, Conwit R, Schoenfeld D, Keroack M, Felsenstein D, Krivickas L, David WS, Vriesendorp F, Pestronk A, Caress JB, Katz J, Simpson E, Rosenfeld J, Pascuzzi R, Glass J, Rezania K, Rothstein JD, Greenblatt DJ, Cudkowicz ME, Northeast ALS Consortium. Design and initial results of a multi-phase randomized trial of ceftriaxone in amyotrophic lateral sclerosis. PLoS One 8: e61177, 2013. - PMC - PubMed
-
- Bradesi S, Schwetz I, Ennes HS, Lamy CM, Ohning G, Fanselow M, Pothoulakis C, McRoberts JA, Mayer EA. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol 289: G42–G53, 2005. - PubMed
-
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53: 55–63, 1994. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

