Evidence for a mitochondrial angiotensin-(1-7) system in the kidney
- PMID: 26697984
- PMCID: PMC4824145
- DOI: 10.1152/ajprenal.00479.2015
Evidence for a mitochondrial angiotensin-(1-7) system in the kidney
Abstract
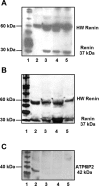
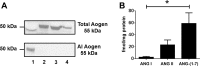
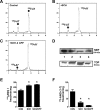
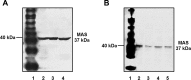
Evidence for an intracellular renin-angiotensin system (RAS) in various cell organelles now includes the endoplasmic reticulum, nucleus, and mitochondria (Mito). Indeed, angiotensin (ANG) AT1 and AT2 receptor subtypes were functionally linked to Mito respiration and nitric oxide production, respectively, in previous studies. We undertook a biochemical analysis of the Mito RAS from male and female sheep kidney cortex. Mito were isolated by differential centrifugation followed by a discontinuous Percoll gradient and were coenriched in Mito membrane markers VDAC and ATP synthase, but not β-actin or cathepsin B. Two distinct renin antibodies identified a 37-kDa protein band in Mito; angiotensinogen (Aogen) conversion was abolished by the inhibitor aliskiren. Mito Aogen was detected by an Aogen antibody to an internal sequence of the protein, but not with an antibody directed against the ANG I N terminus. ANG peptides were quantified by three direct RIAs; mitochondrial ANG II and ANG-(1-7) contents were higher compared with ANG I (23 ± 8 and 58 ± 17 vs. 2 ± 1 fmol/mg protein; P < 0.01, n = 3). 125I-ANG I metabolism primarily revealed the formation of 125I-ANG-(1-7) in Mito that reflects the endopeptidases neprilysin and thimet oligopeptidase. Last, immunoblot studies utilizing the ANG-(1-7)/Mas receptor antibody revealed the protein in isolated Mito from sheep renal cortex. Collectively, the current data demonstrate that Mito actively metabolize the RAS precursor protein Aogen, suggesting that ANG-(1-7) may be generated within Mito to establish an intramitochondrial RAS tone and contribute to renal mitochondrial function.
Keywords: ANG-(1–7); angiotensin-(1–7); kidney; mitochondria; renin-angiotensin system.
Copyright © 2016 the American Physiological Society.
Figures


Similar articles
-
Nuclear expression of renin-angiotensin system components in NRK-52E renal epithelial cells.J Renin Angiotensin Aldosterone Syst. 2015 Dec;16(4):1135-48. doi: 10.1177/1470320313515039. Epub 2014 Jun 24. J Renin Angiotensin Aldosterone Syst. 2015. PMID: 24961503 Free PMC article.
-
Nuclear angiotensin-(1-7) receptor is functionally coupled to the formation of nitric oxide.Am J Physiol Renal Physiol. 2010 Nov;299(5):F983-90. doi: 10.1152/ajprenal.00371.2010. Epub 2010 Sep 1. Am J Physiol Renal Physiol. 2010. PMID: 20810609 Free PMC article.
-
Effects of renin-angiotensin system blockade on renal angiotensin-(1-7) forming enzymes and receptors.Kidney Int. 2005 Nov;68(5):2189-96. doi: 10.1111/j.1523-1755.2005.00675.x. Kidney Int. 2005. PMID: 16221218
-
Angiotensin-(1-7): a bioactive fragment of the renin-angiotensin system.Regul Pept. 1998 Nov 30;78(1-3):13-8. doi: 10.1016/s0167-0115(98)00134-7. Regul Pept. 1998. PMID: 9879742 Review.
-
Angiotensin-(1-7) and its effects in the kidney.ScientificWorldJournal. 2009 Jun 30;9:522-35. doi: 10.1100/tsw.2009.70. ScientificWorldJournal. 2009. PMID: 19578709 Free PMC article. Review.
Cited by
-
Mitochondrial angiotensin receptors and cardioprotective pathways.Am J Physiol Heart Circ Physiol. 2019 Jun 1;316(6):H1426-H1438. doi: 10.1152/ajpheart.00772.2018. Epub 2019 Apr 12. Am J Physiol Heart Circ Physiol. 2019. PMID: 30978131 Free PMC article. Review.
-
Angiotensinogen import in isolated proximal tubules: evidence for mitochondrial trafficking and uptake.Am J Physiol Renal Physiol. 2017 May 1;312(5):F879-F886. doi: 10.1152/ajprenal.00246.2016. Epub 2016 Nov 30. Am J Physiol Renal Physiol. 2017. PMID: 27903492 Free PMC article.
-
Identification of dipeptidyl peptidase 3 as the Angiotensin-(1-7) degrading peptidase in human HK-2 renal epithelial cells.Peptides. 2016 Sep;83:29-37. doi: 10.1016/j.peptides.2016.06.005. Epub 2016 Jun 15. Peptides. 2016. PMID: 27315786 Free PMC article.
-
Molecular Mechanisms of Proteins - Targets for SARS-CoV-2 (Review).Sovrem Tekhnologii Med. 2021;12(6):98-108. doi: 10.17691/stm2020.12.6.11. Epub 2020 Dec 28. Sovrem Tekhnologii Med. 2021. PMID: 34796023 Free PMC article. Review.
-
The Renin-Angiotensin-Aldosterone System as a Therapeutic Target in Late Injury Caused by Ischemia-Reperfusion.Int J Endocrinol. 2018 Apr 4;2018:3614303. doi: 10.1155/2018/3614303. eCollection 2018. Int J Endocrinol. 2018. PMID: 29849615 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

