Activation of mTOR (mechanistic target of rapamycin) in rheumatic diseases
- PMID: 26698023
- PMCID: PMC5314913
- DOI: 10.1038/nrrheum.2015.172
Activation of mTOR (mechanistic target of rapamycin) in rheumatic diseases
Abstract
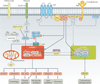
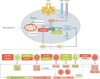
Mechanistic target of rapamycin (mTOR, also known as mammalian target of rapamycin) is a ubiquitous serine/threonine kinase that regulates cell growth, proliferation and survival. These effects are cell-type-specific, and are elicited in response to stimulation by growth factors, hormones and cytokines, as well as to internal and external metabolic cues. Rapamycin was initially developed as an inhibitor of T-cell proliferation and allograft rejection in the organ transplant setting. Subsequently, its molecular target (mTOR) was identified as a component of two interacting complexes, mTORC1 and mTORC2, that regulate T-cell lineage specification and macrophage differentiation. mTORC1 drives the proinflammatory expansion of T helper (TH) type 1, TH17, and CD4(-)CD8(-) (double-negative, DN) T cells. Both mTORC1 and mTORC2 inhibit the development of CD4(+)CD25(+)FoxP3(+) T regulatory (TREG) cells and, indirectly, mTORC2 favours the expansion of T follicular helper (TFH) cells which, similarly to DN T cells, promote B-cell activation and autoantibody production. In contrast to this proinflammatory effect of mTORC2, mTORC1 favours, to some extent, an anti-inflammatory macrophage polarization that is protective against infections and tissue inflammation. Outside the immune system, mTORC1 controls fibroblast proliferation and chondrocyte survival, with implications for tissue fibrosis and osteoarthritis, respectively. Rapamycin (which primarily inhibits mTORC1), ATP-competitive, dual mTORC1/mTORC2 inhibitors and upstream regulators of the mTOR pathway are being developed to treat autoimmune, hyperproliferative and degenerative diseases. In this regard, mTOR blockade promises to increase life expectancy through treatment and prevention of rheumatic diseases.
Figures
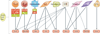
References
-
- Vezina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J. Antibiot. (Tokyo) 1975;28:721–726. - PubMed
-
- Sehgal SN, Bansback CC. Rapamycin: in vitro profile of a new immunosuppressive macrolide. Ann. NY Acad. Sci. 1993;685:58–67. - PubMed
-
- Collier DSJ, et al. Rapamycin in experimental renal allografts in dogs and pigs. Transplant. Proc. 1990;22:1674–1675. - PubMed
-
- Calne RY, et al. Rapamycin for immunosuppression in organ allografting. Lancet. 1989;334:227. - PubMed
-
- Warner LM, Adams LM, Sehgal SN. Rapamycin prolongs survival and arrests pathophysiologic changes in murine systemic lupus erythematosus. Arthritis Rheum. 1994;37:289–297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

