Effects of Pre-Natal Vitamin D Supplementation with Partial Correction of Vitamin D Deficiency on Early Life Healthcare Utilisation: A Randomised Controlled Trial
- PMID: 26698303
- PMCID: PMC4689556
- DOI: 10.1371/journal.pone.0145303
Effects of Pre-Natal Vitamin D Supplementation with Partial Correction of Vitamin D Deficiency on Early Life Healthcare Utilisation: A Randomised Controlled Trial
Abstract
Background: Some observational studies have suggested that higher prenatal Vitamin D intake may be associated with improved health outcomes in childhood. However there have been mixed results in this area with some negative studies, especially for effects on atopic and respiratory outcomes. We examined the effect of prenatal Vitamin D on healthcare utilisation in the first three years of life.
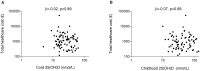
Methods: In an ethnically stratified randomised controlled trial conducted at St Mary's Hospital London, 180 women at 27 weeks gestation were allocated to no Vitamin D, 800 IU ergocalciferol daily until delivery, or a single oral bolus of 200,000 IU cholecalciferol. Participants were randomised in blocks of 15 using computer-generated numbers and investigators were blinded to group assignment. Supplementation increased maternal and cord blood 25(OH) vitamin D concentrations, but levels remained lower than current recommendations. Primary health economic outcome was overall cost of unscheduled healthcare utilisation in the first three years of life as documented in the child's electronic health record. Secondary outcomes included cost attributable to: primary and secondary healthcare visits, respiratory and atopic complaints, cost in years 1, 2 and 3 of life and cost and frequency of prescribed medication. All costs were calculated as pounds sterling. Differences between groups were analysed using unpaired t-test or Mann-Whitney U test, and analysis of variance for adjusted analyses.
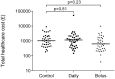
Results: We assessed 99/180 (55%) complete electronic health records, control (n = 31), daily (n = 36) and bolus (n = 32). We found no difference in total healthcare utilisation costs between the control and daily (mean difference in costs in pounds sterling 1.02, 95%CI -1.60, 1.65; adjusted 1.07, 95%CI -1.62, 1.86) or control and bolus groups (mean difference -1.58, 95%CI -2.63, 1.06; adjusted -1.40, 95%CI -2.45, 1.24). There were no adverse effects of supplementation reported during the trial.
Conclusions: We found no evidence that prenatal vitamin D supplementation from 27 weeks gestation to delivery, at doses which failed to completely correct maternal vitamin D deficiency, influence overall healthcare utilisation in children in the first 3 years.
Trial registration: Controlled-Trials.com ISRCTN68645785.
Conflict of interest statement
Figures
Similar articles
-
Maternal vitamin D supplementation during pregnancy and lactation to promote infant growth in Dhaka, Bangladesh (MDIG trial): study protocol for a randomized controlled trial.Trials. 2015 Jul 14;16:300. doi: 10.1186/s13063-015-0825-8. Trials. 2015. PMID: 26169781 Free PMC article. Clinical Trial.
-
Prenatal vitamin d supplementation and child respiratory health: a randomised controlled trial.PLoS One. 2013 Jun 24;8(6):e66627. doi: 10.1371/journal.pone.0066627. Print 2013. PLoS One. 2013. PMID: 23826104 Free PMC article. Clinical Trial.
-
VITALITY trial: protocol for a randomised controlled trial to establish the role of postnatal vitamin D supplementation in infant immune health.BMJ Open. 2015 Dec 16;5(12):e009377. doi: 10.1136/bmjopen-2015-009377. BMJ Open. 2015. PMID: 26674499 Free PMC article. Clinical Trial.
-
Vitamin D during pregnancy: why observational studies suggest deficiency and interventional studies show no improvement in clinical outcomes? A narrative review.J Endocrinol Invest. 2015 Dec;38(12):1265-75. doi: 10.1007/s40618-015-0363-y. Epub 2015 Jul 29. J Endocrinol Invest. 2015. PMID: 26219612 Review.
-
Vitamin D and Allergy Susceptibility during Gestation and Early Life.Nutrients. 2021 Mar 21;13(3):1015. doi: 10.3390/nu13031015. Nutrients. 2021. PMID: 33801051 Free PMC article. Review.
Cited by
-
25-Hydroxyvitamin D supplementation and health-service utilization for upper respiratory tract infection in young children.Public Health Nutr. 2017 Jul;20(10):1816-1824. doi: 10.1017/S1368980017000921. Epub 2017 Jun 5. Public Health Nutr. 2017. PMID: 28578751 Free PMC article.
-
Vitamin D supplementation for women during pregnancy.Cochrane Database Syst Rev. 2019 Jul 26;7(7):CD008873. doi: 10.1002/14651858.CD008873.pub4. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2024 Jul 30;7:CD008873. doi: 10.1002/14651858.CD008873.pub5. PMID: 31348529 Free PMC article. Updated.
-
Vitamin D supplementation for women during pregnancy.Cochrane Database Syst Rev. 2024 Jul 30;7(7):CD008873. doi: 10.1002/14651858.CD008873.pub5. Cochrane Database Syst Rev. 2024. PMID: 39077939 Free PMC article.
References
-
- Scragg R, Camargo CA Jr. Frequency of leisure-time physical activity and serum 25-hydroxyvitamin D levels in the US population: results from the Third National Health and Nutrition Examination Survey. American journal of epidemiology. 2008;168(6):577–86; discussion 87–91. 10.1093/aje/kwn163 - DOI - PMC - PubMed
-
- Wang SS, Hon KL, Kong AP, Pong HN, Wong GW, Leung TF. Vitamin D deficiency is associated with diagnosis and severity of childhood atopic dermatitis. Pediatric allergy and immunology: official publication of the European Society of Pediatric Allergy and Immunology. 2014;25(1):30–5. 10.1111/pai.12167 . - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

