Glycolipids produced by Rouxiella sp. DSM 100043 and isolation of the biosurfactants via foam-fractionation
- PMID: 26698314
- PMCID: PMC4689721
- DOI: 10.1186/s13568-015-0167-7
Glycolipids produced by Rouxiella sp. DSM 100043 and isolation of the biosurfactants via foam-fractionation
Abstract
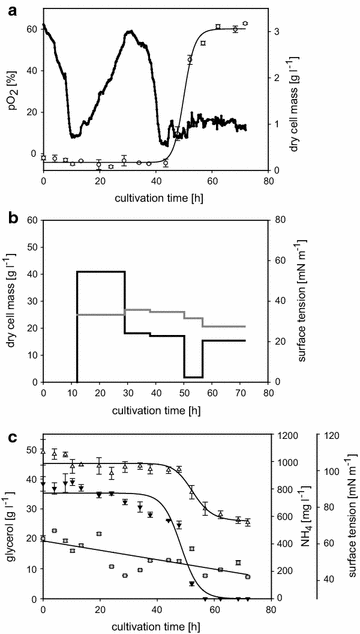
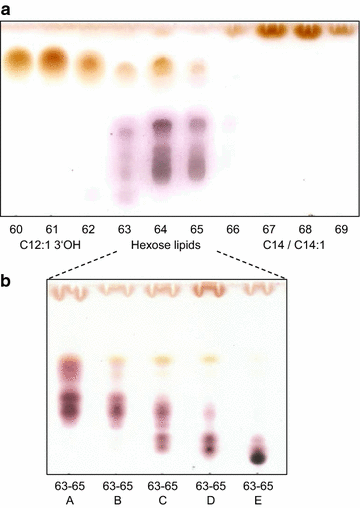
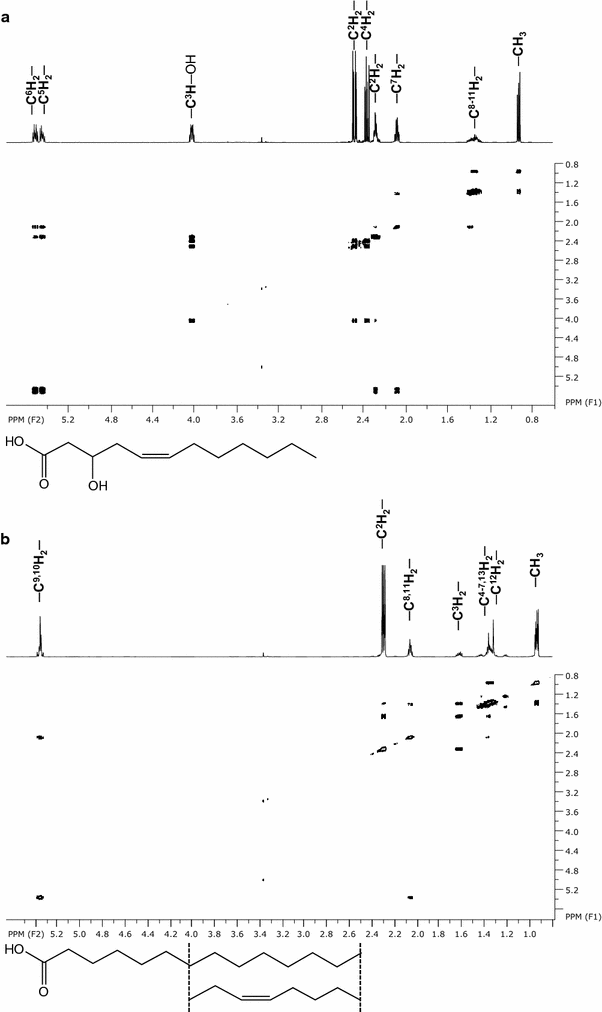
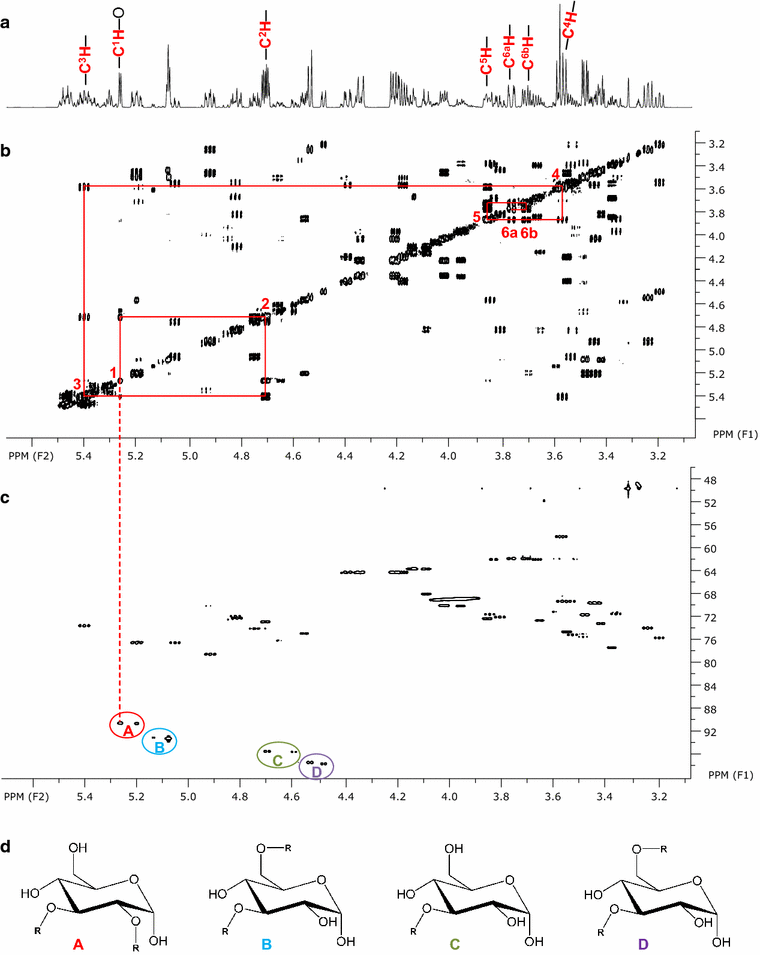
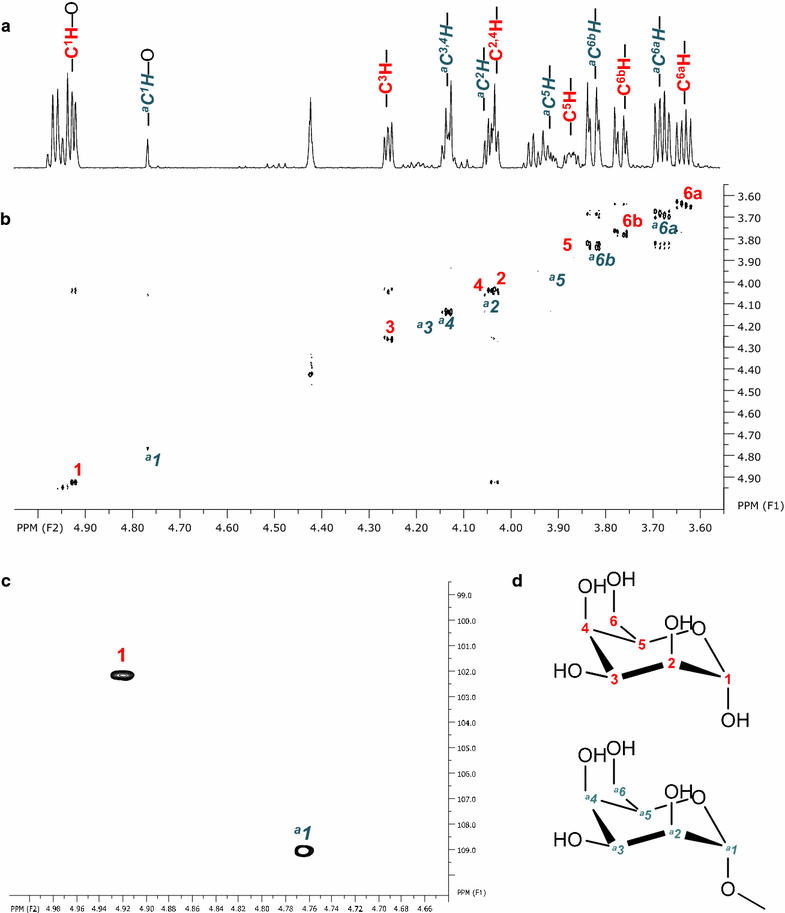
Microorganisms produce a great variety of secondary metabolites that feature surface active and bioactive properties. Those possessing an amphiphilc molecular structure are also termed biosurfactant and are of great interest due to their often unique properties. Rouxiella sp. DSM 100043 is a gram negative enterobacter isolated from peat-bog soil and described as a new biosurfactant producing species in this study. Rouxiella sp. produces glycolipids, biosurfactants with a carbohydrate moiety in its structure. This study characterizes the composition of glycolipids with different hydrophobicities that have been produced during cultivation in a bioreactor and been extracted and purified from separated foam. Using two dimensional nuclear magnetic resonance spectroscopy, the hydrophilic moieties are elucidated as glucose with various acylation sites and as talose within the most polar glycolipids. The presence of 3' hydroxy lauroleic acid as well as myristic and myristoleic acid has been detected.
Keywords: Biosurfactant; Emulsifier; Glycolipid; Hydroxy linoleic acid; Myristic acid; Myristoleic acid; Rouxiella; Serratia; Surfactant; Talose.
Figures
References
-
- Chen CY, Baker SC, Darton RC. Batch production of biosurfactant with foam fractionation. J Chem Technol Biotechnol. 2006;81:1923–1931. doi: 10.1002/jctb.1625. - DOI
-
- Hausmann R, Syldatk C (2014) Types and classification of microbial surfactants. In: Kosaric, Vardar-Sukan (eds) Biosurfactants: production and utilization—processes, technologies, and economics. London, New York: CRC Press, Taylor & Francis Group, Boca Raton, vol 159, pp 165.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

