Coexpression of cellulases in Pichia pastoris as a self-processing protein fusion
- PMID: 26698316
- PMCID: PMC4689727
- DOI: 10.1186/s13568-015-0170-z
Coexpression of cellulases in Pichia pastoris as a self-processing protein fusion
Abstract
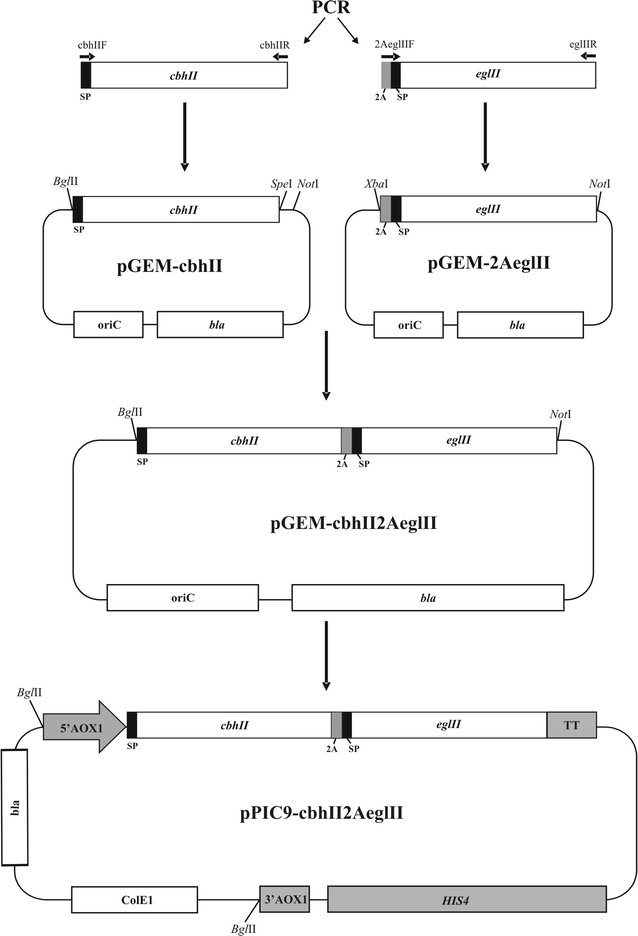
The term cellulase refers to any component of the enzymatic complex produced by some fungi, bacteria and protozoans which act serially or synergistically to catalyze the cleavage of cellulosic materials. Cellulases have been widely used in many industrial applications ranging from food industry to the production of second generation ethanol. In an effort to develop new strategies to minimize the costs of enzyme production we describe the development of a Pichia pastoris strain able to coproduce two different cellulases. For that purpose the eglII (endoglucanase II) and cbhII (cellobiohydrolase II) genes from Trichoderma reesei were fused in-frame separated by the self-processing 2A peptide sequence from the foot-and-mouth disease virus. The protein fusion construct was placed under the control of the strong inducible AOX1 promoter. Analysis of culture supernatants from methanol-induced yeast transformants showed that the protein fusion was effectively processed. Enzymatic assay showed that the processed enzymes were fully functional with the same catalytic properties of the individual enzymes produced separately. Furthermore, when combined both enzymes acted synergistically on filter paper to produce cellobiose as the main end-product. Based on these results we propose that P. pastoris should be considered as an alternative platform for the production of cellulases at competitive costs.
Keywords: 2A peptide; Cellobiohydrolase; Endoglucanase; Pichia pastoris; Protein fusion.
Figures





Similar articles
-
Comparison of the heterologous expression of Trichoderma reesei endoglucanase II and cellobiohydrolase II in the yeasts Pichia pastoris and Yarrowia lipolytica.Mol Biotechnol. 2013 Jun;54(2):158-69. doi: 10.1007/s12033-012-9557-0. Mol Biotechnol. 2013. PMID: 22638966
-
Revisiting overexpression of a heterologous β-glucosidase in Trichoderma reesei: fusion expression of the Neosartorya fischeri Bgl3A to cbh1 enhances the overall as well as individual cellulase activities.Microb Cell Fact. 2016 Jul 11;15(1):122. doi: 10.1186/s12934-016-0520-9. Microb Cell Fact. 2016. PMID: 27400964 Free PMC article.
-
Characterization and high level expression of acidic endoglucanase in Pichia pastoris.Appl Biochem Biotechnol. 2014 Feb;172(4):2253-65. doi: 10.1007/s12010-013-0672-6. Epub 2013 Dec 19. Appl Biochem Biotechnol. 2014. PMID: 24347161
-
Heterologous Protein Expression in Pichia pastoris: Latest Research Progress and Applications.Chembiochem. 2018 Jan 4;19(1):7-21. doi: 10.1002/cbic.201700460. Epub 2017 Dec 13. Chembiochem. 2018. PMID: 29235217 Review.
-
Pichia pastoris Promoters.Methods Mol Biol. 2019;1923:97-112. doi: 10.1007/978-1-4939-9024-5_3. Methods Mol Biol. 2019. PMID: 30737736 Review.
Cited by
-
Post-translational control of genetic circuits using Potyvirus proteases.Nucleic Acids Res. 2016 Jul 27;44(13):6493-502. doi: 10.1093/nar/gkw537. Epub 2016 Jun 13. Nucleic Acids Res. 2016. PMID: 27298256 Free PMC article.
-
Prediction of strain engineerings that amplify recombinant protein secretion through the machine learning approach MaLPHAS.Eng Biol. 2022 Sep 16;6(4):82-90. doi: 10.1049/enb2.12025. eCollection 2022 Dec. Eng Biol. 2022. PMID: 36968340 Free PMC article.
-
Co-expression of endoglucanase and cellobiohydrolase from yak rumen in lactic acid bacteria and its preliminary application in whole-plant corn silage fermentation.Front Microbiol. 2024 Sep 17;15:1442797. doi: 10.3389/fmicb.2024.1442797. eCollection 2024. Front Microbiol. 2024. PMID: 39355421 Free PMC article.
-
ATNT: an enhanced system for expression of polycistronic secondary metabolite gene clusters in Aspergillus niger.Fungal Biol Biotechnol. 2017 Dec 19;4:13. doi: 10.1186/s40694-017-0042-1. eCollection 2017. Fungal Biol Biotechnol. 2017. PMID: 29270299 Free PMC article.
References
-
- Boonvitthya N, Bozonnet S, Burapatana V, O’Donohue MJ, Chulalaksananukul W. Comparison of the heterologous expression of Trichoderma reesei endoglucanase II and cellobiohydrolase II in the yeasts Pichia pastoris and Yarrowia lipolytica. Mol Biotechnol. 2013;54:158–169. doi: 10.1007/s12033-012-9557-0. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

