Evaluation of options for harvest of a recombinant E. Coli fermentation producing a domain antibody using ultra scale-down techniques and pilot-scale verification
- PMID: 26698375
- PMCID: PMC4991298
- DOI: 10.1002/btpr.2220
Evaluation of options for harvest of a recombinant E. Coli fermentation producing a domain antibody using ultra scale-down techniques and pilot-scale verification
Abstract
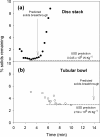
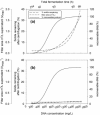
Ultra scale-down (USD) methods operating at the millilitre scale were used to characterise full-scale processing of E. coli fermentation broths autolysed to different extents for release of a domain antibody. The focus was on the primary clarification stages involving continuous centrifugation followed by depth filtration. The performance of this sequence was predicted by USD studies to decrease significantly with increased extents of cell lysis. The use of polyethyleneimine reagent was studied to treat the lysed cell broth by precipitation of soluble contaminants such as DNA and flocculation of cell debris material. The USD studies were used to predict the impact of this treatment on the performance and here it was found that the fermentation could be run to maximum productivity using an acceptable clarification process (e.g., a centrifugation stage operating at 0.11 L/m(2) equivalent gravity settling area per hour followed by a resultant required depth filter area of 0.07 m(2) /L supernatant). A range of USD predictions was verified at the pilot scale for centrifugation followed by depth filtration. © 2015 American Institute of Chemical Engineers Biotechnol. Prog., 32:382-392, 2016.
Keywords: E. coli; centrifugation; domain antibody; fermentation; filtration; flocculation.
© 2015 American Institute of Chemical Engineers.
Figures








Similar articles
-
An ultra scale-down approach to study the interaction of fermentation, homogenization, and centrifugation for antibody fragment recovery from rec E. coli.Biotechnol Bioeng. 2013 Aug;110(8):2150-60. doi: 10.1002/bit.24891. Epub 2013 May 7. Biotechnol Bioeng. 2013. PMID: 23475508
-
Adapted ultra scale-down approach for predicting the centrifugal separation behavior of high cell density cultures.Biotechnol Prog. 2007 Nov-Dec;23(6):1404-10. doi: 10.1021/bp070175d. Epub 2007 Oct 20. Biotechnol Prog. 2007. PMID: 17949106
-
Ultra scale-down prediction using microwell technology of the industrial scale clarification characteristics by centrifugation of mammalian cell broths.Biotechnol Bioeng. 2009 Oct 1;104(2):321-31. doi: 10.1002/bit.22393. Biotechnol Bioeng. 2009. PMID: 19507199
-
Manufacturing of Proteins and Antibodies: Chapter Downstream Processing Technologies.Adv Biochem Eng Biotechnol. 2018;165:95-114. doi: 10.1007/10_2016_54. Adv Biochem Eng Biotechnol. 2018. PMID: 28776064 Review.
-
Clarification of vaccines: An overview of filter based technology trends and best practices.Biotechnol Adv. 2016 Jan-Feb;34(1):1-13. doi: 10.1016/j.biotechadv.2015.11.005. Epub 2015 Dec 2. Biotechnol Adv. 2016. PMID: 26657051 Review.
Cited by
-
Detecting cell lysis using viscosity monitoring in E. coli fermentation to prevent product loss.Biotechnol Prog. 2016 Jul 8;32(4):1069-76. doi: 10.1002/btpr.2292. Epub 2016 May 17. Biotechnol Prog. 2016. PMID: 27111912 Free PMC article.
-
Flocculant Screening Method at Lab Scale for Application in Disc Stack Centrifuges with Hermetic Design.Chem Eng Technol. 2018 Dec;41(12):2312-2322. doi: 10.1002/ceat.201800202. Epub 2018 Oct 30. Chem Eng Technol. 2018. PMID: 31007398 Free PMC article.
-
Downstream Processing of Chlamydomonas reinhardtii TN72 for Recombinant Protein Recovery.Front Bioeng Biotechnol. 2019 Dec 6;7:383. doi: 10.3389/fbioe.2019.00383. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31867315 Free PMC article.
-
Recent Developments in Bioprocessing of Recombinant Proteins: Expression Hosts and Process Development.Front Bioeng Biotechnol. 2019 Dec 20;7:420. doi: 10.3389/fbioe.2019.00420. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31921823 Free PMC article. Review.
References
-
- Huang C Jr., Lin H, Yang X. Industrial production of recombinant therapeutics in Escherichia coli and its recent advancements. J Ind Microbiol Biotechnol. 2012;39:383–399. - PubMed
-
- Kaufmann M. Unstable proteins: how to subject them to chromatographic separations for purification procedures. J Chromatogr B Biomed Sci Appl. 1997;699:347–369. - PubMed
-
- Rinas U, Hoffmann F. Selective leakage of Host‐Cell Proteins during high‐cell‐density cultivation of recombinant and non‐recombinant Escherichia coli . Biotechnol Prog. 2004;20:679–687. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

