Non-canonical actions of mismatch repair
- PMID: 26698648
- PMCID: PMC4740236
- DOI: 10.1016/j.dnarep.2015.11.020
Non-canonical actions of mismatch repair
Abstract
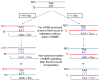
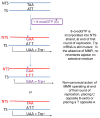
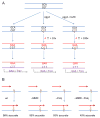
At the heart of the mismatch repair (MMR) system are proteins that recognize mismatches in DNA. Such mismatches can be mispairs involving normal or damaged bases or insertion/deletion loops due to strand misalignment. When such mispairs are generated during replication or recombination, MMR will direct removal of an incorrectly paired base or block recombination between nonidentical sequences. However, when mispairs are recognized outside the context of replication, proper strand discrimination between old and new DNA is lost, and MMR can act randomly and mutagenically on mispaired DNA. Such non-canonical actions of MMR are important in somatic hypermutation and class switch recombination, expansion of triplet repeats, and potentially in mutations arising in nondividing cells. MMR involvement in damage recognition and signaling is complex, with the end result likely dependent on the amount of DNA damage in a cell.
Keywords: DNA damage; DNA replication fidelity; Mispaired base; Mutagenesis; Replication; Strand discrimination.
Copyright © 2015 Elsevier B.V. All rights reserved.
Conflict of interest statement
The author declares that there are no conflicts of interest.
Figures

Similar articles
-
Mismatch repair-dependent mutagenesis in nondividing cells.Proc Natl Acad Sci U S A. 2012 Apr 17;109(16):6153-8. doi: 10.1073/pnas.1115361109. Epub 2012 Apr 2. Proc Natl Acad Sci U S A. 2012. PMID: 22474380 Free PMC article.
-
Influence of oxidized purine processing on strand directionality of mismatch repair.J Biol Chem. 2015 Apr 17;290(16):9986-99. doi: 10.1074/jbc.M114.629907. Epub 2015 Feb 18. J Biol Chem. 2015. PMID: 25694431 Free PMC article.
-
Mismatch repair balances leading and lagging strand DNA replication fidelity.PLoS Genet. 2012;8(10):e1003016. doi: 10.1371/journal.pgen.1003016. Epub 2012 Oct 11. PLoS Genet. 2012. PMID: 23071460 Free PMC article.
-
Postreplicative mismatch repair.Cold Spring Harb Perspect Biol. 2013 Apr 1;5(4):a012633. doi: 10.1101/cshperspect.a012633. Cold Spring Harb Perspect Biol. 2013. PMID: 23545421 Free PMC article. Review.
-
Exonuclease 1-dependent and independent mismatch repair.DNA Repair (Amst). 2015 Aug;32:24-32. doi: 10.1016/j.dnarep.2015.04.010. Epub 2015 Apr 30. DNA Repair (Amst). 2015. PMID: 25956862 Free PMC article. Review.
Cited by
-
Counteraction of Oxidative Stress by Vitamin E Affects Epigenetic Regulation by Increasing Global Methylation and Gene Expression of MLH1 and DNMT1 Dose Dependently in Caco-2 Cells.Oxid Med Cell Longev. 2018 Mar 22;2018:3734250. doi: 10.1155/2018/3734250. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 29854080 Free PMC article.
-
Close encounters: Moving along bumps, breaks, and bubbles on expanded trinucleotide tracts.DNA Repair (Amst). 2017 Aug;56:144-155. doi: 10.1016/j.dnarep.2017.06.017. Epub 2017 Jun 9. DNA Repair (Amst). 2017. PMID: 28690053 Free PMC article. Review.
-
RNA/DNA Binding Protein TDP43 Regulates DNA Mismatch Repair Genes with Implications for Genome Stability.bioRxiv [Preprint]. 2024 Nov 8:2024.05.16.594552. doi: 10.1101/2024.05.16.594552. bioRxiv. 2024. PMID: 38798341 Free PMC article. Preprint.
-
CDK-independent role of D-type cyclins in regulating DNA mismatch repair.Mol Cell. 2024 Apr 4;84(7):1224-1242.e13. doi: 10.1016/j.molcel.2024.02.010. Epub 2024 Mar 7. Mol Cell. 2024. PMID: 38458201 Free PMC article.
-
Antibody diversification caused by disrupted mismatch repair and promiscuous DNA polymerases.DNA Repair (Amst). 2016 Feb;38:110-116. doi: 10.1016/j.dnarep.2015.11.011. Epub 2015 Dec 2. DNA Repair (Amst). 2016. PMID: 26719140 Free PMC article. Review.
References
-
- Li GM. Mechanisms and functions of DNA mismatch repair. Cell Res. 2008;18:85–98. - PubMed
-
- Jiricny J. The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol. 2006;7:335–346. - PubMed
-
- Iyer RR, Pluciennik A, Burdett V, Modrich PL. DNA mismatch repair: functions and mechanisms. Chem Rev. 2006;106:302–323. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

