Baseline Serum Osteopontin Levels Predict the Clinical Effectiveness of Tocilizumab but Not Infliximab in Biologic-Naïve Patients with Rheumatoid Arthritis: A Single-Center Prospective Study at 1 Year (the Keio First-Bio Cohort Study)
- PMID: 26698858
- PMCID: PMC4689361
- DOI: 10.1371/journal.pone.0145468
Baseline Serum Osteopontin Levels Predict the Clinical Effectiveness of Tocilizumab but Not Infliximab in Biologic-Naïve Patients with Rheumatoid Arthritis: A Single-Center Prospective Study at 1 Year (the Keio First-Bio Cohort Study)
Erratum in
-
Correction: Baseline Serum Osteopontin Levels Predict the Clinical Effectiveness of Tocilizumab but Not Infliximab in Biologic-Naïve Patients with Rheumatoid Arthritis: A Single-Center Prospective Study at 1 Year (the Keio First-Bio Cohort Study).PLoS One. 2016 Mar 21;11(3):e0152341. doi: 10.1371/journal.pone.0152341. eCollection 2016. PLoS One. 2016. PMID: 26999019 Free PMC article. No abstract available.
Abstract
Objective: To explore the baseline predictors of clinical effectiveness after tocilizumab or infliximab treatment in biologic-naïve rheumatoid arthritis patients.
Methods: Consecutive biologic-naïve patients with rheumatoid arthritis initiating infliximab (n = 57) or tocilizumab (n = 70) treatment were included in our prospective cohort study. Our cohort started in February 2010, and the patients observed for at least 1 year as of April 2013 were analysed. We assessed baseline variables including patients' characteristics (age, sex, disease duration, prednisolone dose, methotrexate dose, other disease-modifying antirheumatic drug use, Clinical Disease Activity Index [CDAI]) and serum biomarker levels (C-reactive protein, immunoglobulin M-rheumatoid factor, anti-cyclic citrullinated protein/peptide antibodies, interferon-γ, interleukin (IL)-1β, IL-2, IL-6, IL-8, IL-10, IL-17, tumor necrosis factor-α, soluble intercellular adhesion molecule-1, bone alkaline phosphatase, osteonectin, osteopontin) to extract factors associated with clinical remission (CDAI ≤ 2.8) at 1 year using univariate analyses, and the extracted factors were entered into a multivariate logistic regression model. Similar analyses were also performed for Simplified Disease Activity Index (SDAI) remission (≤ 3.3) and Disease Activity Score with 28 joint counts, erythrocyte sedimentation rate (DAS28-ESR) remission (< 2.6).
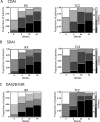
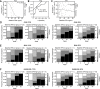
Results: There were no significant differences in the baseline characteristics except for methotrexate use between the groups. In the multivariate analyses, the low baseline osteopontin levels (OR 0.9145, 95% CI 0.8399-0.9857) were identified as predictors of CDAI remission in the tocilizumab group, whereas no predictors of CDAI remission were found in the infliximab group. Similar results were obtained when using SDAI and DAS28-ESR remission criteria.
Conclusion: Baseline low serum osteopontin levels predict clinical remission 1 year after tocilizumab treatment and not infliximab treatment in biologic-naïve patients with rheumatoid arthritis. Our prediction model provided insights into how to optimize the choice of biologics and warrants external validation in other cohorts.
Conflict of interest statement
Figures
References
-
- Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:907–16. - PubMed
-
- McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–42. - PubMed
-
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

