Ibuprofen versus fosfomycin for uncomplicated urinary tract infection in women: randomised controlled trial
- PMID: 26698878
- PMCID: PMC4688879
- DOI: 10.1136/bmj.h6544
Ibuprofen versus fosfomycin for uncomplicated urinary tract infection in women: randomised controlled trial
Abstract
Study question: Can treatment of the symptoms of uncomplicated urinary tract infection (UTI) with ibuprofen reduce the rate of antibiotic prescriptions without a significant increase in symptoms, recurrences, or complications?
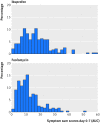
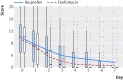
Methods: Women aged 18-65 with typical symptoms of UTI and without risk factors or complications were recruited in 42 German general practices and randomly assigned to treatment with a single dose of fosfomycin 3 g (n=246; 243 analysed) or ibuprofen 3 × 400 mg (n=248; 241 analysed) for three days (and the respective placebo dummies in both groups). In both groups additional antibiotic treatment was subsequently prescribed as necessary for persistent, worsening, or recurrent symptoms. The primary endpoints were the number of all courses of antibiotic treatment on days 0-28 (for UTI or other conditions) and burden of symptoms on days 0-7. The symptom score included dysuria, frequency/urgency, and low abdominal pain.
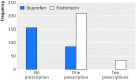
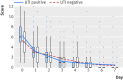
Study answer and limitations: The 248 women in the ibuprofen group received significantly fewer course of antibiotics, had a significantly higher total burden of symptoms, and more had pyelonephritis. Four serious adverse events occurred that lead to hospital referrals; one of these was potentially related to the trial drug. Results have to be interpreted carefully as they might apply to women with mild to moderate symptoms rather than to all those with an uncomplicated UTI.
What this paper adds: Two thirds of women with uncomplicated UTI treated symptomatically with ibuprofen recovered without any antibiotics. Initial symptomatic treatment is a possible approach to be discussed with women willing to avoid immediate antibiotics and to accept a somewhat higher burden of symptoms.
Funding, competing interests, data sharing: German Federal Ministry of Education and Research (BMBF) No 01KG1105. Patient level data are available from the corresponding author. Patient consent was not obtained but the data are anonymised and risk of identification is low.Trial registration No ClinicalTrialGov Identifier NCT01488955.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: All authors have completed the uniform disclosure form at
Figures




Comment in
-
Which treatment strategy for women with symptoms of urinary tract infection?BMJ. 2015 Dec 29;351:h6888. doi: 10.1136/bmj.h6888. BMJ. 2015. PMID: 26715541 Free PMC article.
-
Fosfomycin should not be first line treatment for uncomplicated urinary tract infection.BMJ. 2016 Jan 28;352:i413. doi: 10.1136/bmj.i413. BMJ. 2016. PMID: 26823000 No abstract available.
-
Authors' reply to Al-Wali and Hughes.BMJ. 2016 Jan 28;352:i415. doi: 10.1136/bmj.i415. BMJ. 2016. PMID: 26823520 No abstract available.
-
Non-antibiotic effects of ibuprofen in uncomplicated urinary tract infection in women.BMJ. 2016 Mar 2;352:i1171. doi: 10.1136/bmj.i1171. BMJ. 2016. PMID: 26936586 No abstract available.
-
Authors' reply to Kristiansen.BMJ. 2016 Mar 2;352:i1172. doi: 10.1136/bmj.i1172. BMJ. 2016. PMID: 26936753 No abstract available.
-
Antibiotika bei Harnwegsinfekten oft unnötig.MMW Fortschr Med. 2016 Jun 9;158(11):38. doi: 10.1007/s15006-016-8372-0. MMW Fortschr Med. 2016. PMID: 27271405 German. No abstract available.
References
-
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013. www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf.
-
- World Health Organization. Antimicrobial resistance: Global Report on Surveillance 2014 2014. http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
