Nuclear deformability and telomere dynamics are regulated by cell geometric constraints
- PMID: 26699462
- PMCID: PMC4711833
- DOI: 10.1073/pnas.1513189113
Nuclear deformability and telomere dynamics are regulated by cell geometric constraints
Abstract
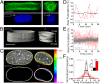
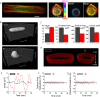
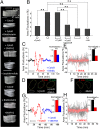
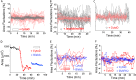
Forces generated by the cytoskeleton can be transmitted to the nucleus and chromatin via physical links on the nuclear envelope and the lamin meshwork. Although the role of these active forces in modulating prestressed nuclear morphology has been well studied, the effect on nuclear and chromatin dynamics remains to be explored. To understand the regulation of nuclear deformability by these active forces, we created different cytoskeletal states in mouse fibroblasts using micropatterned substrates. We observed that constrained and isotropic cells, which lack long actin stress fibers, have more deformable nuclei than elongated and polarized cells. This nuclear deformability altered in response to actin, myosin, formin perturbations, or a transcriptional down-regulation of lamin A/C levels in the constrained and isotropic geometry. Furthermore, to probe the effect of active cytoskeletal forces on chromatin dynamics, we tracked the spatiotemporal dynamics of heterochromatin foci and telomeres. We observed increased dynamics and decreased correlation of the heterochromatin foci and telomere trajectories in constrained and isotropic cell geometry. The observed enhanced dynamics upon treatment with actin depolymerizing reagents in elongated and polarized geometry were regained once the reagent was washed off, suggesting an inherent structural memory in chromatin organization. We conclude that active forces from the cytoskeleton and rigidity from lamin A/C nucleoskeleton can together regulate nuclear and chromatin dynamics. Because chromatin remodeling is a necessary step in transcription control and its memory, genome integrity, and cellular deformability during migration, our results highlight the importance of cell geometric constraints as critical regulators in cell behavior.
Keywords: actomyosin contractility; cell geometry; chromatin dynamics; mechanotransduction; telomere dynamics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

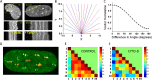
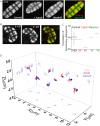
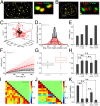

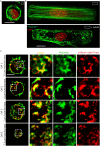

References
-
- Woringer M, Darzacq X, Izeddin I. Geometry of the nucleus: A perspective on gene expression regulation. Curr Opin Chem Biol. 2014;20:112–119. - PubMed
-
- Burke B, Stewart CL. Functional architecture of the cell’s nucleus in development, aging, and disease. Curr Top Dev Biol. 2014;109:1–52. - PubMed
-
- Broers JL, et al. Decreased mechanical stiffness in LMNA-/- cells is caused by defective nucleo-cytoskeletal integrity: Implications for the development of laminopathies. Hum Mol Genet. 2004;13(21):2567–2580. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

