Bottlenecks and selective sweeps during domestication have increased deleterious genetic variation in dogs
- PMID: 26699508
- PMCID: PMC4711855
- DOI: 10.1073/pnas.1512501113
Bottlenecks and selective sweeps during domestication have increased deleterious genetic variation in dogs
Abstract
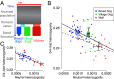
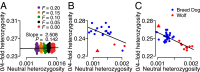
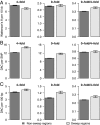
Population bottlenecks, inbreeding, and artificial selection can all, in principle, influence levels of deleterious genetic variation. However, the relative importance of each of these effects on genome-wide patterns of deleterious variation remains controversial. Domestic and wild canids offer a powerful system to address the role of these factors in influencing deleterious variation because their history is dominated by known bottlenecks and intense artificial selection. Here, we assess genome-wide patterns of deleterious variation in 90 whole-genome sequences from breed dogs, village dogs, and gray wolves. We find that the ratio of amino acid changing heterozygosity to silent heterozygosity is higher in dogs than in wolves and, on average, dogs have 2-3% higher genetic load than gray wolves. Multiple lines of evidence indicate this pattern is driven by less efficient natural selection due to bottlenecks associated with domestication and breed formation, rather than recent inbreeding. Further, we find regions of the genome implicated in selective sweeps are enriched for amino acid changing variants and Mendelian disease genes. To our knowledge, these results provide the first quantitative estimates of the increased burden of deleterious variants directly associated with domestication and have important implications for selective breeding programs and the conservation of rare and endangered species. Specifically, they highlight the costs associated with selective breeding and question the practice favoring the breeding of individuals that best fit breed standards. Our results also suggest that maintaining a large population size, rather than just avoiding inbreeding, is a critical factor for preventing the accumulation of deleterious variants.
Keywords: bottleneck; deleterious mutations; domestication; selective sweep.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Lohmueller KE. The distribution of deleterious genetic variation in human populations. Curr Opin Genet Dev. 2014;29:139–146. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

