Lipid Membranes Facilitate Conformational Changes Required for Reovirus Cell Entry
- PMID: 26699639
- PMCID: PMC4810687
- DOI: 10.1128/JVI.02997-15
Lipid Membranes Facilitate Conformational Changes Required for Reovirus Cell Entry
Abstract
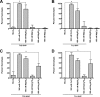
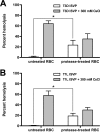
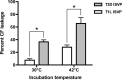
Cellular entry of nonenveloped and enveloped viruses is often accompanied by dramatic conformational changes within viral structural proteins. These rearrangements are triggered by a variety of mechanisms, such as low pH, virus-receptor interactions, and virus-host chaperone interactions. Reoviruses, a model system for entry of nonenveloped viruses, undergo a series of disassembly steps within the host endosome. One of these steps, infectious subviral particle (ISVP)-to-ISVP* conversion, is necessary for delivering the genome-containing viral core into host cells, but the physiological trigger that mediates ISVP-to-ISVP* conversion during cell entry is unknown. Structural studies of the reovirus membrane penetration protein, μ1, predict that interactions between μ1 and negatively charged lipid head groups may promote ISVP* formation; however, experimental evidence for this idea is lacking. Here, we show that the presence of polyanions (SO4(2-) and HPO4(2-)) or lipids in the form of liposomes facilitates ISVP-to-ISVP* conversion. The requirement for charged lipids appears to be selective, since phosphatidylcholine and phosphatidylethanolamine promoted ISVP* formation, whereas other lipids, such as sphingomyelin and sulfatide, either did not affect ISVP* formation or prevented ISVP* formation. Thus, our work provides evidence that interactions with membranes can function as a trigger for a nonenveloped virus to gain entry into host cells.
Importance: Cell entry, a critical stage in the virus life cycle, concludes with the delivery of the viral genetic material across host membranes. Regulated structural transitions within nonenveloped and enveloped viruses are necessary for accomplishing this step; these conformational changes are predominantly triggered by low pH and/or interactions with host proteins. In this work, we describe a previously unknown trigger, interactions with lipid membranes, which can induce the structural rearrangements required for cell entry. This mechanism operates during entry of mammalian orthoreoviruses. We show that interactions between reovirus entry intermediates and lipid membranes devoid of host proteins promote conformational changes within the viral outer capsid that lead to membrane penetration. Thus, this work illustrates a novel strategy that nonenveloped viruses can use to gain access into cells and how viruses usurp disparate host factors to initiate infection.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures






References
-
- Stegmann T, Booy FP, Wilschut J. 1987. Effects of low pH on influenza virus. Activation and inactivation of the membrane fusion capacity of the hemagglutinin. J Biol Chem 262:17744–17749. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

