Critical Role for Telomerase in the Mechanism of Flow-Mediated Dilation in the Human Microcirculation
- PMID: 26699654
- PMCID: PMC4772813
- DOI: 10.1161/CIRCRESAHA.115.307918
Critical Role for Telomerase in the Mechanism of Flow-Mediated Dilation in the Human Microcirculation
Abstract
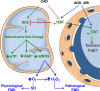
Rationale: Telomerase is a nuclear regulator of telomere elongation with recent reports suggesting a role in regulation of mitochondrial reactive oxygen species. Flow-mediated dilation in patients with cardiovascular disease is dependent on the formation of reactive oxygen species.
Objective: We examined the hypothesis that telomerase activity modulates microvascular flow-mediated dilation, and loss of telomerase activity contributes to the change of mediator from nitric oxide to mitochondrial hydrogen peroxide in patients with coronary artery disease (CAD).
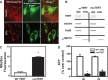
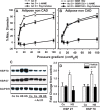
Methods and results: Human coronary and adipose arterioles were isolated for videomicroscopy. Flow-mediated dilation was measured in vessels pretreated with the telomerase inhibitor BIBR-1532 or vehicle. Statistical differences between groups were determined using a 2-way analysis of variance repeated measure (n≥4; P<0.05). L-NAME (N(ω)-nitro-L-arginine methyl ester; nitric oxide synthase inhibitor) abolished flow-mediated dilation in arterioles from subjects without CAD, whereas polyethylene glycol-catalase (PEG-catalase; hydrogen peroxide scavenger) had no effect. After exposure to BIBR-1532, arterioles from non-CAD subjects maintained the magnitude of dilation but changed the mediator from nitric oxide to mitochondrial hydrogen peroxide (% max diameter at 100 cm H2O: vehicle 74.6±4.1, L-NAME 37.0±2.0*, PEG-catalase 82.1±2.8; BIBR-1532 69.9±4.0, L-NAME 84.7±2.2, PEG-catalase 36.5±6.9*). Conversely, treatment of microvessels from CAD patients with the telomerase activator AGS 499 converted the PEG-catalase-inhibitable dilation to one mediated by nitric oxide (% max diameter at 100 cm H2O: adipose, AGS 499 78.5±3.9; L-NAME 10.9±17.5*; PEG-catalase 79.2±4.9). Endothelial-independent dilation was not altered with either treatment.
Conclusions: We have identified a novel role for telomerase in re-establishing a physiological mechanism of vasodilation in arterioles from subjects with CAD. These findings suggest a new target for reducing the oxidative milieu in the microvasculature of patients with CAD.
Keywords: coronary artery disease; flow-mediated dilation; microvascular dysfunction; mitochondria; reactive oxygen species; telomerase activity; vascular biology.
© 2015 The Authors.
Figures




Comment in
-
The Secret Life of Telomerase.Circ Res. 2016 Mar 4;118(5):781-2. doi: 10.1161/CIRCRESAHA.116.308290. Circ Res. 2016. PMID: 26941421 No abstract available.
References
-
- Aviv H, Khan MY, Skurnick J, Okuda K, Kimura M, Gardner J, Priolo L, Aviv A. Age dependent aneuploidy and telomere length of the human vascular endothelium. Atherosclerosis. 2001;159:281–287. - PubMed
-
- Brouilette S, Singh RK, Thompson JR, Goodall AH, Samani NJ. White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol. 2003;23:842–846. doi: 10.1161/01.ATV.0000067426.96344.32. - PubMed
-
- Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation. 2002;105:1541–1544. - PubMed
-
- Shaik S, Inuzuka H, Liu P, Wei W, Wang Z. Endothelium Aging and Vascular Diseases: INTECH Open Access Publisher. 2013.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

