PI3 kinase mutations and mutational load as poor prognostic markers in diffuse glioma patients
- PMID: 26699864
- PMCID: PMC4690424
- DOI: 10.1186/s40478-015-0265-4
PI3 kinase mutations and mutational load as poor prognostic markers in diffuse glioma patients
Abstract
Introduction: Recent advances in molecular diagnostics allow diffuse gliomas to be classified based on their genetic changes into distinct prognostic subtypes. However, a systematic analysis of all molecular markers has thus far not been performed; most classification schemes use a predefined and select set of genes/molecular markers. Here, we have analysed the TCGA dataset (combined glioblastoma (GBM) and lower grade glioma (LGG) datasets) to identify all prognostic genetic markers in diffuse gliomas in order to generate a comprehensive classification scheme.
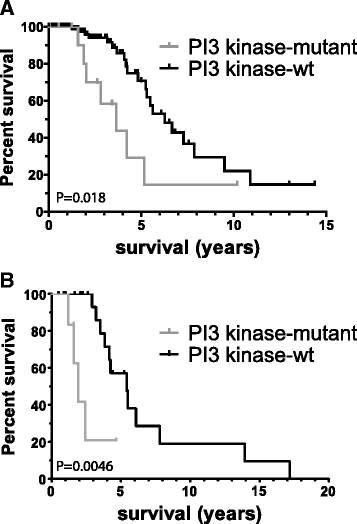
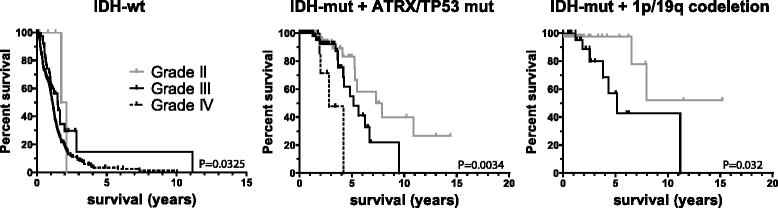
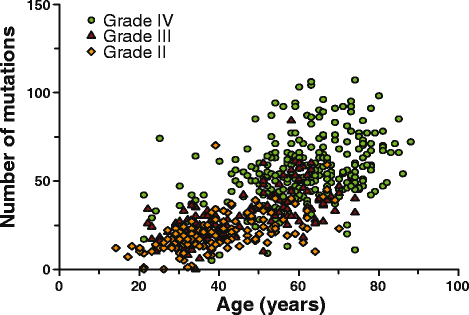
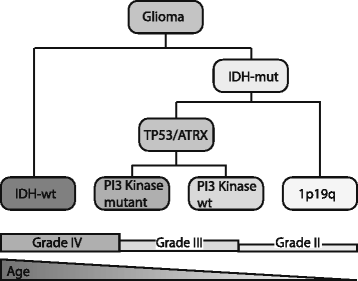
Results: Of the molecular markers investigated (all genes mutated at a population frequency >1.7 % and frequent chromosomal imbalances) in the entire glioma dataset, 57 were significantly associated with overall survival. Of these, IDH1 or IDH2 mutations are associated with lowest hazard ratio, which confirms IDH as the most important prognostic marker in diffuse gliomas. Subsequent subgroup analysis largely confirms many of the currently used molecular classification schemes for diffuse gliomas (ATRX or TP53 mutations, 1p19q codeletion). Our analysis also identified PI3-kinase mutations as markers of poor prognosis in IDH-mutated + ATRX/TP53 mutated diffuse gliomas, median survival 3.7 v. 6.3 years (P = 0.02, Hazard rate (HR) 2.93, 95 % confidence interval (CI) 1.16 - 7.38). PI3-kinase mutations were also prognostic in two independent datasets. In our analysis, no additional molecular markers were identified that further refine the molecular classification of diffuse gliomas. Interestingly, these molecular classifiers do not fully explain the variability in survival observed for diffuse glioma patients. We demonstrate that tumor grade remains an important prognostic factor for overall survival in diffuse gliomas, even within molecular glioma subtypes. Tumor grade was correlated with the mutational load (the number of non-silent mutations) of the tumor: grade II diffuse gliomas harbour fewer genetic changes than grade III or IV, even within defined molecular subtypes (e.g. ATRX mutated diffuse gliomas).
Conclusion: We have identified PI3K mutations as novel prognostic markers in gliomas. We also demonstrate that the mutational load is associated with tumor grade. The increase in mutational load may partially explain the increased aggressiveness of higher grade diffuse gliomas when a subset of the affected genes actively contributes to gliomagenesis and/or progression.
Figures
Similar articles
-
IDH mutation, 1p19q codeletion and ATRX loss in WHO grade II gliomas.Oncotarget. 2015 Oct 6;6(30):30295-305. doi: 10.18632/oncotarget.4497. Oncotarget. 2015. PMID: 26210286 Free PMC article.
-
Use of telomerase promoter mutations to mark specific molecular subsets with reciprocal clinical behavior in IDH mutant and IDH wild-type diffuse gliomas.J Neurosurg. 2018 Apr;128(4):1102-1114. doi: 10.3171/2016.11.JNS16973. Epub 2017 Jun 16. J Neurosurg. 2018. PMID: 28621624
-
DNA methylation signatures for 2016 WHO classification subtypes of diffuse gliomas.Clin Epigenetics. 2017 Apr 4;9:32. doi: 10.1186/s13148-017-0331-9. eCollection 2017. Clin Epigenetics. 2017. PMID: 28392842 Free PMC article.
-
Histomolecular classification of adult diffuse gliomas: the diagnostic value of immunohistochemical markers.Rev Neurol (Paris). 2011 Oct;167(10):683-90. doi: 10.1016/j.neurol.2011.07.006. Epub 2011 Sep 1. Rev Neurol (Paris). 2011. PMID: 21889777 Review.
-
Biomarker-driven diagnosis of diffuse gliomas.Mol Aspects Med. 2015 Nov;45:87-96. doi: 10.1016/j.mam.2015.05.002. Epub 2015 May 21. Mol Aspects Med. 2015. PMID: 26004297 Review.
Cited by
-
CNS cancer immunity cycle and strategies to target this for glioblastoma.Oncotarget. 2018 Apr 27;9(32):22802-22816. doi: 10.18632/oncotarget.24896. eCollection 2018 Apr 27. Oncotarget. 2018. PMID: 29854316 Free PMC article. Review.
-
Durable complete responses in some recurrent high-grade glioma patients treated with Toca 511 + Toca FC.Neuro Oncol. 2018 Sep 3;20(10):1383-1392. doi: 10.1093/neuonc/noy075. Neuro Oncol. 2018. PMID: 29762717 Free PMC article. Clinical Trial.
-
Advanced immunotherapies for glioblastoma: tumor neoantigen vaccines in combination with immunomodulators.Acta Neuropathol Commun. 2023 May 10;11(1):79. doi: 10.1186/s40478-023-01569-y. Acta Neuropathol Commun. 2023. PMID: 37165457 Free PMC article. Review.
-
Mutational burden, immune checkpoint expression, and mismatch repair in glioma: implications for immune checkpoint immunotherapy.Neuro Oncol. 2017 Aug 1;19(8):1047-1057. doi: 10.1093/neuonc/nox026. Neuro Oncol. 2017. PMID: 28371827 Free PMC article.
-
Identifying driver modules based on multi-omics biological networks in prostate cancer.IET Syst Biol. 2022 Dec;16(6):187-200. doi: 10.1049/syb2.12050. Epub 2022 Aug 30. IET Syst Biol. 2022. PMID: 36039671 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

