Prevalence of AAV1 neutralizing antibodies and consequences for a clinical trial of gene transfer for advanced heart failure
- PMID: 26699914
- PMCID: PMC6558655
- DOI: 10.1038/gt.2015.109
Prevalence of AAV1 neutralizing antibodies and consequences for a clinical trial of gene transfer for advanced heart failure
Abstract
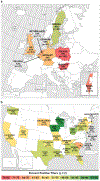
Adeno-associated virus serotype 1 (AAV1) has many advantages as a gene therapy vector, but the presence of pre-existing neutralizing antibodies (NAbs) is an important limitation. This study was designed to determine: (1) characteristics of AAV NAbs in human subjects, (2) prevalence of AAV1 NAbs in heart failure patients and (3) utility of aggressive immunosuppressive therapy in reducing NAb seroconversion in an animal model. NAb titers were assessed in a cohort of heart failure patients and in patients screened for a clinical trial of gene therapy with AAV1 carrying the sarcoplasmic reticulum calcium ATPase gene (AAV1/SERCA2a). AAV1 NAbs were found in 59.5% of 1552 heart failure patients. NAb prevalence increased with age (P=0.001) and varied geographically. The pattern of NAb titers suggested that exposure is against AAV2, with AAV1 NAb seropositivity due to crossreactivity. The effects of immunosuppression on NAb formation were tested in mini-pigs treated with immunosuppressant therapy before, during and after a single AAV1/SERCA2a infusion. Aggressive immunosuppression did not prevent formation of AAV1 NAbs. We conclude that immunosuppression is unlikely to be a viable solution for repeat AAV1 dosing. Strategies to reduce NAbs in heart failure patients are needed to increase eligibility for gene transfer using AAV vectors.
Conflict of interest statement
CONFLICT OF INTEREST
This study was funded by Celladon Corporation (San Diego, CA, USA). Drs Greenberg, Butler, Felker, Ponikowski, Voors and Hajjar have received compensation for consultancies or board memberships from Celladon Corporation, the sponsor of this study. Drs Pogoda, Provost, Guerrero and Zsebo are former employees of Celladon.
Figures
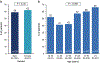
References
-
- Du L, Kido M, Lee DV, Rabinowitz JE, Samulski RJ, Jamieson SW et al. Differential myocardial gene delivery by recombinant serotype-specific adeno-associated viral vectors. Mol Ther 2004; 10: 604–608. - PubMed
-
- Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac diseases (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum CA2 +-ATPase in patients with advanced heart failure. Circulation 2011; 124: 304–313. - PMC - PubMed
-
- Zsebo K, Yaroshinsky A, Rudy JJ, Wagner K, Greenberg B, Jessup M et al. Long-term effects of AAV1/SERCA2a gene transfer in patients with severe heart failure. Analysis of recurrent cardiovascular events and mortality. Circ Res 2014; 114: 101–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

