Involvement of Rac1 signalling pathway in the development and maintenance of acute inflammatory pain induced by bee venom injection
- PMID: 26700000
- PMCID: PMC4761090
- DOI: 10.1111/bph.13413
Involvement of Rac1 signalling pathway in the development and maintenance of acute inflammatory pain induced by bee venom injection
Abstract
Background and purpose: The Rho GTPase, Rac1, is involved in the pathogenesis of neuropathic pain induced by malformation of dendritic spines in the spinal dorsal horn (sDH) neurons. In the present study, the contribution of spinal Rac1 to peripheral inflammatory pain was studied.
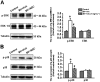
Experimental approach: Effects of s.c. bee venom (BV) injection on cellular localization of Rac1 in the rat sDH was determined with double labelling immunofluorescence. Activation of Rac1 and its downstream effector p21-activated kinase (PAK), ERKs and p38 MAPK in inflammatory pain states was evaluated with a pull-down assay and Western blotting. The preventive and therapeutic analgesic effects of intrathecal administration of NSC23766, a selective inhibitor of Rac1, on BV-induced spontaneous nociception and pain hypersensitivity were investigated.
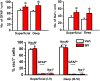
Key results: Rac1 labelling was mainly localized within neurons in both the superficial and deep layers of the sDH in rats of naïve, vehicle-treated and inflamed (BV injected) groups. GTP-Rac1-PAK and ERKs/p38 were activated following s.c. BV injection. Post-treatment with intrathecal NSC23766 significantly inhibited GTP-Rac1 activity and phosphorylation of Rac1-PAK, ERKs and p38 MAPK in the sDH. Both pre-treatment and post-treatment with intrathecal NSC23766 dose-dependently attenuated the paw flinches, primary thermal and mechanical hyperalgesia and the mirror-image thermal hyperalgesia induced by BV injection, but without affecting the baseline pain sensitivity and motor coordination.
Conclusions and implications: The spinal GTP-Rac1-PAK-ERK/p38MAPK signalling pathway is involved in both the development and maintenance of peripheral inflammatory pain and can be used as a potential molecular target for developing a novel therapeutic strategy for clinical pain.
© 2015 The British Pharmacological Society.
Figures







References
-
- Cao FL, Liu MG, Hao J, Li Z, Lu ZM, Chen J (2007). Different roles of spinal p38 and c‐Jun. N‐terminal kinase pathways in bee venom‐induced multiple pain‐related behaviors. Neurosci Lett 427: 50–54. - PubMed
-
- Chen HS, Chen J (2000). Secondary heat, but not mechanical, hyperalgesia induced by subcutaneous injection of bee venom in the conscious rat: effect of systemic MK‐801, a non‐competitive NMDA receptor antagonist. Eur J Pain 4: 389–401. - PubMed
-
- Chen HS, Chen J, Sun YY (2000). Contralateral heat hyperalgesia induced by unilaterally intraplantar bee venom injection is produced by central changes: a behavioral study in the conscious rat. Neurosci Lett 284: 45–48. - PubMed
-
- Chen HS, He X, Qu F, Kang SM, Yu Y, Liao D, et al (2009). Differential roles of peripheral mitogen‐activated protein kinase signal transduction pathways in bee venom‐induced nociception and inflammation in conscious rats. J Pain 10: 201–207. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

