Microbial Surface Colonization and Biofilm Development in Marine Environments
- PMID: 26700108
- PMCID: PMC4711185
- DOI: 10.1128/MMBR.00037-15
Microbial Surface Colonization and Biofilm Development in Marine Environments
Abstract
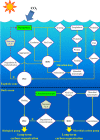
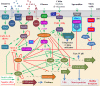
Biotic and abiotic surfaces in marine waters are rapidly colonized by microorganisms. Surface colonization and subsequent biofilm formation and development provide numerous advantages to these organisms and support critical ecological and biogeochemical functions in the changing marine environment. Microbial surface association also contributes to deleterious effects such as biofouling, biocorrosion, and the persistence and transmission of harmful or pathogenic microorganisms and their genetic determinants. The processes and mechanisms of colonization as well as key players among the surface-associated microbiota have been studied for several decades. Accumulating evidence indicates that specific cell-surface, cell-cell, and interpopulation interactions shape the composition, structure, spatiotemporal dynamics, and functions of surface-associated microbial communities. Several key microbial processes and mechanisms, including (i) surface, population, and community sensing and signaling, (ii) intraspecies and interspecies communication and interaction, and (iii) the regulatory balance between cooperation and competition, have been identified as critical for the microbial surface association lifestyle. In this review, recent progress in the study of marine microbial surface colonization and biofilm development is synthesized and discussed. Major gaps in our knowledge remain. We pose questions for targeted investigation of surface-specific community-level microbial features, answers to which would advance our understanding of surface-associated microbial community ecology and the biogeochemical functions of these communities at levels from molecular mechanistic details through systems biological integration.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures


References
-
- Söhngen NL. 1913. Einfluss von Kolloïden auf mikrobiologische Prozesse. Centralbl Bakteriol Parasitenkd Infektionskr 38:621–647.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

