Multipotent adult progenitor cells for hypoxic-ischemic injury in the preterm brain
- PMID: 26700169
- PMCID: PMC4690228
- DOI: 10.1186/s12974-015-0459-5
Multipotent adult progenitor cells for hypoxic-ischemic injury in the preterm brain
Abstract
Background: Preterm infants are at risk for hypoxic-ischemic encephalopathy. No therapy exists to treat this brain injury and subsequent long-term sequelae. We have previously shown in a well-established pre-clinical model of global hypoxia-ischemia (HI) that mesenchymal stem cells are a promising candidate for the treatment of hypoxic-ischemic brain injury. In the current study, we investigated the neuroprotective capacity of multipotent adult progenitor cells (MAPC®), which are adherent bone marrow-derived cells of an earlier developmental stage than mesenchymal stem cells and exhibiting more potent anti-inflammatory and regenerative properties.
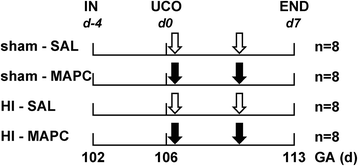
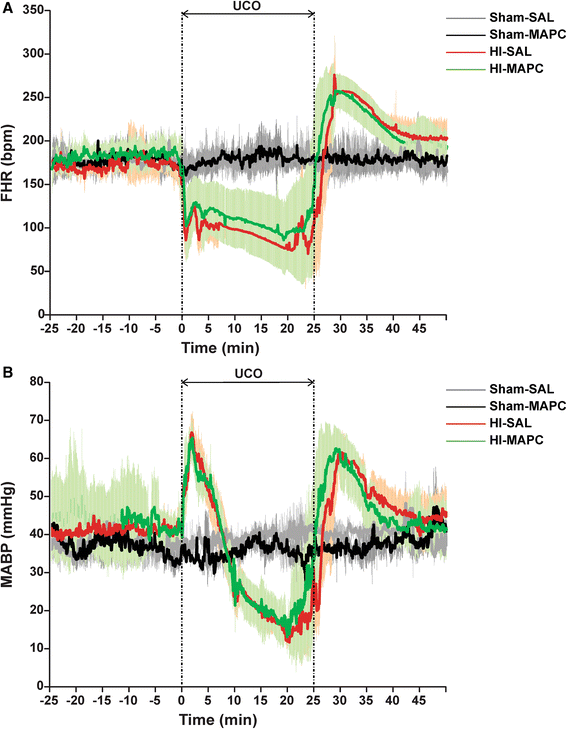
Methods: Instrumented preterm sheep fetuses were subjected to global hypoxia-ischemia by 25 min of umbilical cord occlusion at a gestational age of 106 (term ~147) days. During a 7-day reperfusion period, vital parameters (e.g., blood pressure and heart rate; baroreceptor reflex) and (amplitude-integrated) electroencephalogram were recorded. At the end of the experiment, the preterm brain was studied by histology.
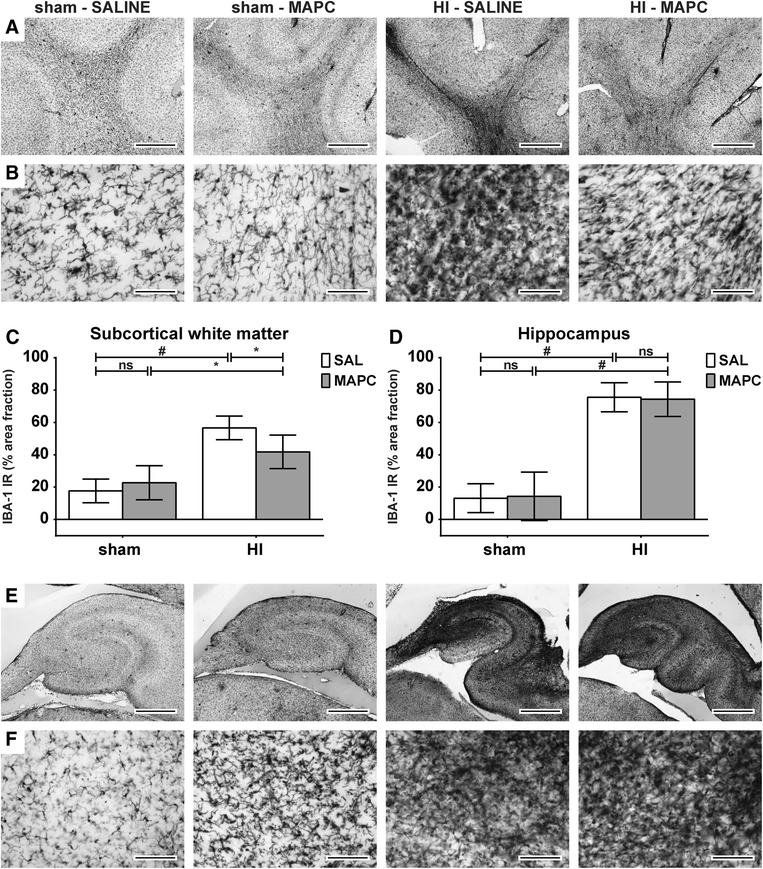
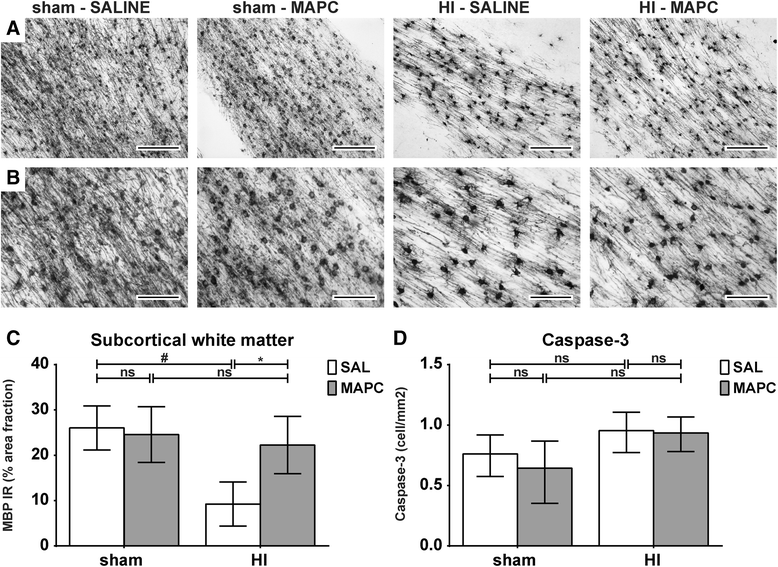
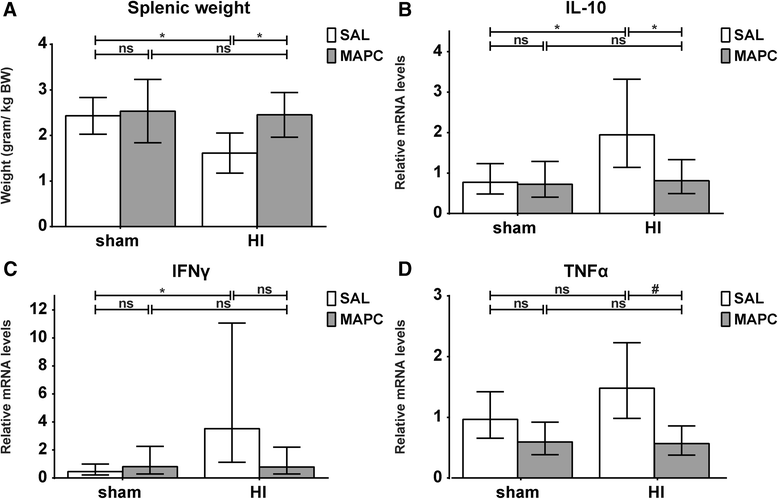
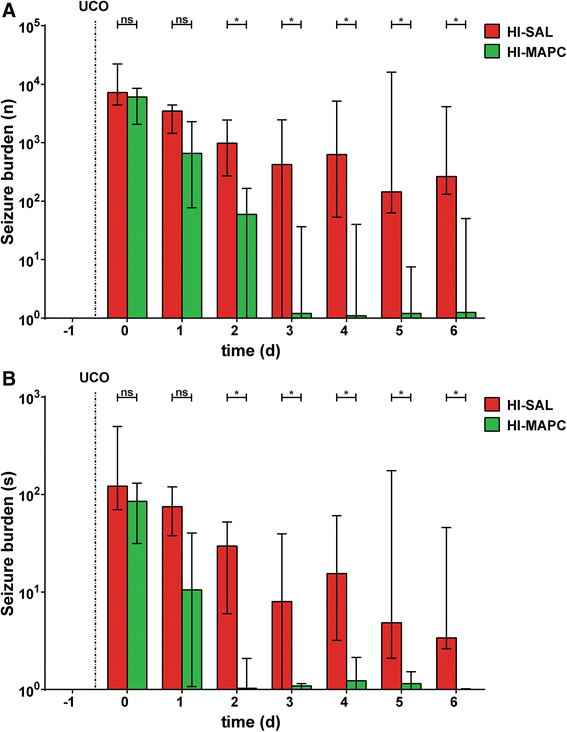
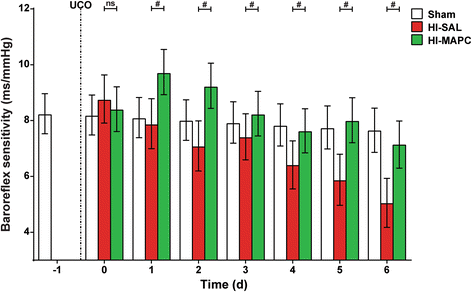
Results: Systemic administration of MAPC therapy reduced the number and duration of seizures and prevented decrease in baroreflex sensitivity after global HI. In addition, MAPC cells prevented HI-induced microglial proliferation in the preterm brain. These anti-inflammatory effects were associated with MAPC-induced prevention of hypomyelination after global HI. Besides attenuation of the cerebral inflammatory response, our findings showed that MAPC cells modulated the peripheral splenic inflammatory response, which has been implicated in the etiology of hypoxic-ischemic injury in the preterm brain.
Conclusions: In a pre-clinical animal model MAPC cell therapy improved the functional and structural outcome of the preterm brain after global HI. Future studies should establish the mechanism and long-term therapeutic effects of neuroprotection established by MAPC cells in the developing preterm brain exposed to HI. Our study may form the basis for future clinical trials, which will evaluate whether MAPC therapy is capable of reducing neurological sequelae in preterm infants with hypoxic-ischemic encephalopathy.
Figures
Similar articles
-
Systemic G-CSF attenuates cerebral inflammation and hypomyelination but does not reduce seizure burden in preterm sheep exposed to global hypoxia-ischemia.Exp Neurol. 2013 Dec;250:293-303. doi: 10.1016/j.expneurol.2013.09.026. Epub 2013 Oct 8. Exp Neurol. 2013. PMID: 24120465
-
Systemic multipotent adult progenitor cells protect the cerebellum after asphyxia in fetal sheep.Stem Cells Transl Med. 2021 Jan;10(1):57-67. doi: 10.1002/sctm.19-0157. Epub 2020 Sep 28. Stem Cells Transl Med. 2021. PMID: 32985793 Free PMC article.
-
Mesenchymal Stromal Cell-Derived Extracellular Vesicles Protect the Fetal Brain After Hypoxia-Ischemia.Stem Cells Transl Med. 2016 Jun;5(6):754-63. doi: 10.5966/sctm.2015-0197. Epub 2016 May 9. Stem Cells Transl Med. 2016. PMID: 27160705 Free PMC article.
-
Models of white matter injury: comparison of infectious, hypoxic-ischemic, and excitotoxic insults.Ment Retard Dev Disabil Res Rev. 2002;8(1):30-8. doi: 10.1002/mrdd.10007. Ment Retard Dev Disabil Res Rev. 2002. PMID: 11921384 Review.
-
Cell therapy for neonatal hypoxia-ischemia and cerebral palsy.Ann Neurol. 2012 May;71(5):589-600. doi: 10.1002/ana.22670. Ann Neurol. 2012. PMID: 22522476 Review.
Cited by
-
Delayed intranasal infusion of human amnion epithelial cells improves white matter maturation after asphyxia in preterm fetal sheep.J Cereb Blood Flow Metab. 2019 Feb;39(2):223-239. doi: 10.1177/0271678X17729954. Epub 2017 Sep 12. J Cereb Blood Flow Metab. 2019. PMID: 28895475 Free PMC article.
-
Preterm Brain Injury, Antenatal Triggers, and Therapeutics: Timing Is Key.Cells. 2020 Aug 10;9(8):1871. doi: 10.3390/cells9081871. Cells. 2020. PMID: 32785181 Free PMC article. Review.
-
Trends in mesenchymal stem cell clinical trials 2004-2018: Is efficacy optimal in a narrow dose range?Stem Cells Transl Med. 2020 Jan;9(1):17-27. doi: 10.1002/sctm.19-0202. Epub 2019 Dec 5. Stem Cells Transl Med. 2020. PMID: 31804767 Free PMC article.
-
A Comparison of Phenotypic and Functional Properties of Mesenchymal Stromal Cells and Multipotent Adult Progenitor Cells.Front Immunol. 2019 Aug 28;10:1952. doi: 10.3389/fimmu.2019.01952. eCollection 2019. Front Immunol. 2019. PMID: 31555259 Free PMC article. Review.
-
Peripheral immune cells and perinatal brain injury: a double-edged sword?Pediatr Res. 2022 Jan;91(2):392-403. doi: 10.1038/s41390-021-01818-7. Epub 2021 Nov 8. Pediatr Res. 2022. PMID: 34750522 Free PMC article. Review.
References
-
- Institute of Medicine, editor . Preterm birth: Causes, Consequences and Prevention. Washington: The National Academies Press; 2007. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

