Regulatory T Cell Infusion Can Enhance Memory T Cell and Alloantibody Responses in Lymphodepleted Nonhuman Primate Heart Allograft Recipients
- PMID: 26700196
- PMCID: PMC4919255
- DOI: 10.1111/ajt.13685
Regulatory T Cell Infusion Can Enhance Memory T Cell and Alloantibody Responses in Lymphodepleted Nonhuman Primate Heart Allograft Recipients
Abstract
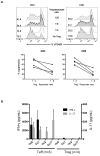
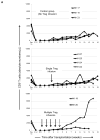
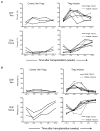
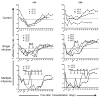
The ability of regulatory T cells (Treg) to prolong allograft survival and promote transplant tolerance in lymphodepleted rodents is well established. Few studies, however, have addressed the therapeutic potential of adoptively transferred, CD4(+) CD25(+) CD127(-) Foxp3(+) (Treg) in clinically relevant large animal models. We infused ex vivo-expanded, functionally stable, nonselected Treg (up to a maximum cumulative dose of 1.87 billion cells) into antithymocyte globulin-lymphodepleted, MHC-mismatched cynomolgus monkey heart graft recipients before homeostatic recovery of effector T cells. The monkeys also received tacrolimus, anti-interleukin-6 receptor monoclonal antibodies and tapered rapamycin maintenance therapy. Treg administration in single or multiple doses during the early postsurgical period (up to 1 month posttransplantation), when host T cells were profoundly depleted, resulted in inferior graft function compared with controls. This was accompanied by increased incidences of effector memory T cells, enhanced interferon-γ production by host CD8(+) T cells, elevated levels of proinflammatory cytokines, and antidonor alloantibodies. The findings caution against infusion of Treg during the early posttransplantation period after lymphodepletion. Despite marked but transient increases in Treg relative to endogenous effector T cells and use of reputed "Treg-friendly" agents, the host environment/immune effector mechanisms instigated under these conditions can perturb rather than favor the potential therapeutic efficacy of adoptively transferred Treg.
Keywords: T cell biology; animal models: nonhuman primate; basic (laboratory) research/science; heart transplantation/cardiology; immune regulation; immunobiology; lymphocyte biology; translational research/science.
© Copyright 2015 The American Society of Transplantation and the American Society of Transplant Surgeons.
Conflict of interest statement
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Figures










Comment in
-
Regulating T Cell Behavior.Am J Transplant. 2016 Jul;16(7):1949-50. doi: 10.1111/ajt.13724. Epub 2016 Mar 17. Am J Transplant. 2016. PMID: 26792650 No abstract available.
References
-
- Bluestone JA, Thomson AW, Shevach EM, Weiner HL. What does the future hold for cell-based tolerogenic therapy? Nat Rev Immunol. 2007;7(8):650–654. - PubMed
-
- Wood KJ, Bushell A, Hester J. Regulatory immune cells in transplantation. Nat Rev Immunol. 2012;12(6):417–430. - PubMed
-
- Juvet SC, Whatcott AG, Bushell AR, Wood KJ. Harnessing regulatory T cells for clinical use in transplantation: the end of the beginning. Am J Transplant. 2014;14(4):750–763. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

