B Cells, Antibodies, and More
- PMID: 26700440
- PMCID: PMC4702236
- DOI: 10.2215/CJN.09430915
B Cells, Antibodies, and More
Abstract
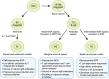
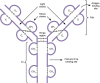
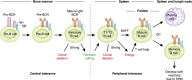
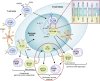
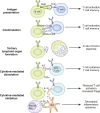
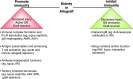
B cells play a central role in the immunopathogenesis of glomerulonephritides and transplant rejection. B cells secrete antibodies that contribute to tissue injury via multiple mechanisms. In addition, B cells contribute to disease pathogenesis in autoimmunity and alloimmunity by presenting antigens as well as providing costimulation and cytokines to T cells. B cells also play an immunomodulatory role in regulating the immune response by secreting cytokines that inhibit disease onset and/or progression. B cell-targeted approaches for treating immune diseases of the kidney and other organs have gained significant momentum. However, much remains to be understood about B-cell biology in order to determine the timing, duration, and context of optimal therapeutic response to B cell-targeted approaches. In this review, we discuss the multifaceted roles of B cells as enhancers and regulators of immunity with relevance to kidney disease and transplantation.
Keywords: Antibodies; B regulatory cells; B-Lymphocytes; T-Lymphocytes; autoimmunity; cytokines; glomerulonephritis; graft rejection; immune system diseases; plasma cells.
Copyright © 2016 by the American Society of Nephrology.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

