β-Cell Deficit in Obese Type 2 Diabetes, a Minor Role of β-Cell Dedifferentiation and Degranulation
- PMID: 26700560
- PMCID: PMC4880126
- DOI: 10.1210/jc.2015-3566
β-Cell Deficit in Obese Type 2 Diabetes, a Minor Role of β-Cell Dedifferentiation and Degranulation
Abstract
Context: Type 2 diabetes is characterized by a β-cell deficit and a progressive defect in β-cell function. It has been proposed that the deficit in β-cells may be due to β-cell degranulation and transdifferentiation to other endocrine cell types.
Objective: The objective of the study was to establish the potential impact of β-cell dedifferentiation and transdifferentiation on β-cell deficit in type 2 diabetes and to consider the alternative that cells with an incomplete identity may be newly forming rather than dedifferentiated.
Design, setting, and participants: Pancreata obtained at autopsy were evaluated from 14 nondiabetic and 13 type 2 diabetic individuals, from four fetal cases, and from 10 neonatal cases.
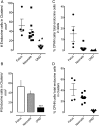
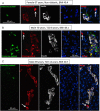
Results: Whereas there was a slight increase in islet endocrine cells expressing no hormone in type 2 diabetes (0.11 ± 0.03 cells/islet vs 0.03 ± 0.01 cells/islet, P < .01), the impact on the β-cell deficit would be minimal. Furthermore, we established that the deficit in β-cells per islet cannot be accounted for by an increase in other endocrine cell types. The distribution of hormone negative endocrine cells in type 2 diabetes (most abundant in cells scattered in the exocrine pancreas) mirrors that in developing (embryo and neonatal) pancreas, implying that these may represent newly forming cells.
Conclusions: Therefore, although we concur that in type 2 diabetes there are endocrine cells with altered cell identity, this process does not account for the deficit in β-cells in type 2 diabetes but may reflect, in part, attempted β-cell regeneration.
Figures




References
-
- Buchanan TA, Xiang AH, Peters RK, et al. Preservation of pancreatic β-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes. 2002;51:2796–2803. - PubMed
-
- Kloppel G, Lohr M, Habich K, Oberholzer M, Heitz PU. Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv Synth Pathol Res. 1985;4:110–125. - PubMed
-
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. β-Cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. - PubMed
-
- Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic β-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab. 2008;10(suppl 4):32–42. - PubMed

