Circadian clocks govern calorie restriction-mediated life span extension through BMAL1- and IGF-1-dependent mechanisms
- PMID: 26700733
- PMCID: PMC4799504
- DOI: 10.1096/fj.15-282475
Circadian clocks govern calorie restriction-mediated life span extension through BMAL1- and IGF-1-dependent mechanisms
Abstract
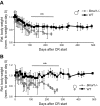
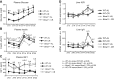
Calorie restriction (CR) increases longevity in many species by unknown mechanisms. The circadian clock was proposed as a potential mediator of CR. Deficiency of the core component of the circadian clock-transcriptional factor BMAL1 (brain and muscle ARNT [aryl hydrocarbon receptor nuclear translocator]-like protein 1)-results in accelerated aging. Here we investigated the role of BMAL1 in mechanisms of CR. The 30% CR diet increased the life span of wild-type (WT) mice by 20% compared to mice on anad libitum(AL) diet but failed to increase life span ofBmal1(-/-)mice. BMAL1 deficiency impaired CR-mediated changes in the plasma levels of IGF-1 and insulin. We detected a statistically significantly reduction of IGF-1 in CRvs.AL by 50 to 70% in WT mice at several daily time points tested, while inBmal1(-/-)the reduction was not significant. Insulin levels in WT were reduced by 5 to 9%, whileBmal1(-/-)induced it by 10 to 35% at all time points tested. CR up-regulated the daily average expression ofBmal1(by 150%) and its downstream target genesPeriods(by 470% forPer1and by 130% forPer2). We propose that BMAL1 is an important mediator of CR, and activation of BMAL1 might link CR mechanisms with biologic clocks.-Patel, S. A., Chaudhari, A., Gupta, R., Velingkaar, N., Kondratov, R. V. Circadian clocks govern calorie restriction-mediated life span extension through BMAL1- and IGF-1-dependent mechanisms.
Keywords: aging; food anticipation; gene expression; glucose; insulin; transcription.
© FASEB.
Figures




References
-
- Ravussin E., Redman L. M., Rochon J., Das S. K., Fontana L., Kraus W. E., Romashkan S., Williamson D. A., Meydani S. N., Villareal D. T., Smith S. R., Stein R. I., Scott T. M., Stewart T. M., Saltzman E., Klein S., Bhapkar M., Martin C. K., Gilhooly C. H., Holloszy J. O., Hadley E. C., Roberts S. B.; CALERIE Study Group (2015) A 2-year randomized controlled trial of human caloric restriction: feasibility and effects on predictors of health span and longevity. J. Gerontol. A Biol. Sci. Med. Sci. 70, 1097–1104 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

