LZTFL1 Upregulated by All-Trans Retinoic Acid during CD4+ T Cell Activation Enhances IL-5 Production
- PMID: 26700766
- PMCID: PMC4724573
- DOI: 10.4049/jimmunol.1500719
LZTFL1 Upregulated by All-Trans Retinoic Acid during CD4+ T Cell Activation Enhances IL-5 Production
Abstract
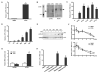
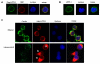
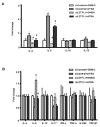
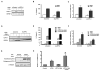
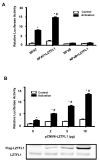
Retinoic acids, which are metabolites of vitamin A, have been shown to be involved in multiple T cell effector responses through their binding to the retinoic acid receptor, a ligand-activated transcription factor. Because the molecular mechanism of regulation by retinoic acid is still not fully uncovered, we investigated the gene expression profile of all-trans retinoic acid (ATRA)-treated human CD4(+) T cells. Leucine zipper transcription factor-like 1 (LZTFL1) was upregulated by ATRA in a dose- and time-dependent manner. The expression of LZTFL1 depended on both ATRA and TCR signaling. LZTFL1 accumulated in the plasma membrane compartment of human CD4(+) T cells, and, during immunological synapse formation, it transiently redistributed to the T cell and APC contact zone, indicating its role in T cell activation. Live-cell imaging demonstrates that at the initial stage of immunological synapse formation, LZTFL1 is concentrated at the APC contact site, and, during later stages, it relocates to the distal pole. Knockdown of LZTFL1 reduced the basal- and ATRA-induced levels of IL-5 in CD4(+) T cells, and overexpression of LZTFL1 enhanced the TCR-mediated NFAT signaling, suggesting that LZTFL1 is an important regulator of ATRA-induced T cell response. Together, these data indicate that LZTFL1 modulates T cell activation and IL-5 levels.
Copyright © 2016 by The American Association of Immunologists, Inc.
Figures

References
-
- De Luca LM. Retinoids and their receptors in differentiation, embryogenesis, and neoplasia. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1991;5:2924–2933. - PubMed
-
- Collins SJ. The role of retinoids and retinoic acid receptors in normal hematopoiesis. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, U.K. 2002;16:1896–1905. - PubMed
-
- Hall JA, Cannons JL, Grainger JR, Dos Santos LM, Hand TW, Naik S, Wohlfert EA, Chou DB, Oldenhove G, Robinson M, Grigg ME, Kastenmayer R, Schwartzberg PL, Belkaid Y. Essential role for retinoic acid in the promotion of CD4(+) T cell effector responses via retinoic acid receptor alpha. Immunity. 2011;34:435–447. - PMC - PubMed
-
- Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

