A LAIR1 insertion generates broadly reactive antibodies against malaria variant antigens
- PMID: 26700814
- PMCID: PMC4869849
- DOI: 10.1038/nature16450
A LAIR1 insertion generates broadly reactive antibodies against malaria variant antigens
Abstract
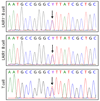
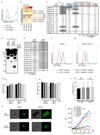
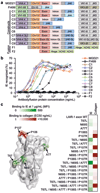
Plasmodium falciparum antigens expressed on the surface of infected erythrocytes are important targets of naturally acquired immunity against malaria, but their high number and variability provide the pathogen with a powerful means of escape from host antibodies. Although broadly reactive antibodies against these antigens could be useful as therapeutics and in vaccine design, their identification has proven elusive. Here we report the isolation of human monoclonal antibodies that recognize erythrocytes infected by different P. falciparum isolates and opsonize these cells by binding to members of the RIFIN family. These antibodies acquired broad reactivity through a novel mechanism of insertion of a large DNA fragment between the V and DJ segments. The insert, which is both necessary and sufficient for binding to RIFINs, encodes the entire 98 amino acid collagen-binding domain of LAIR1, an immunoglobulin superfamily inhibitory receptor encoded on chromosome 19. In each of the two donors studied, the antibodies are produced by a single expanded B-cell clone and carry distinct somatic mutations in the LAIR1 domain that abolish binding to collagen and increase binding to infected erythrocytes. These findings illustrate, with a biologically relevant example, a novel mechanism of antibody diversification by interchromosomal DNA transposition and demonstrate the existence of conserved epitopes that may be suitable candidates for the development of a malaria vaccine.
Figures









Comment in
-
A New Way to Diversify Antibodies by DNA Transposition.Cell. 2016 Feb 11;164(4):601-2. doi: 10.1016/j.cell.2016.01.031. Cell. 2016. PMID: 26871626
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
- 084113/WT_/Wellcome Trust/United Kingdom
- 092741/WT_/Wellcome Trust/United Kingdom
- 077092/WT_/Wellcome Trust/United Kingdom
- 084378/WT_/Wellcome Trust/United Kingdom
- 099811/WT_/Wellcome Trust/United Kingdom
- G1002624/MRC_/Medical Research Council/United Kingdom
- 092654/WT_/Wellcome Trust/United Kingdom
- 250348/ERC_/European Research Council/International
- 084538/WT_/Wellcome Trust/United Kingdom
- 084378/Z/07/A/WT_/Wellcome Trust/United Kingdom
- 084535/WT_/Wellcome Trust/United Kingdom
- 670955/ERC_/European Research Council/International
- 084113/Z/07/Z/WT_/Wellcome Trust/United Kingdom

