A Conserved GPG-Motif in the HIV-1 Nef Core Is Required for Principal Nef-Activities
- PMID: 26700863
- PMCID: PMC4689412
- DOI: 10.1371/journal.pone.0145239
A Conserved GPG-Motif in the HIV-1 Nef Core Is Required for Principal Nef-Activities
Abstract
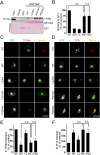
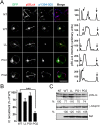
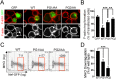
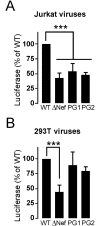
To find out new determinants required for Nef activity we performed a functional alanine scanning analysis along a discrete but highly conserved region at the core of HIV-1 Nef. We identified the GPG-motif, located at the 121-137 region of HIV-1 NL4.3 Nef, as a novel protein signature strictly required for the p56Lck dependent Nef-induced CD4-downregulation in T-cells. Since the Nef-GPG motif was dispensable for CD4-downregulation in HeLa-CD4 cells, Nef/AP-1 interaction and Nef-dependent effects on Tf-R trafficking, the observed effects on CD4 downregulation cannot be attributed to structure constraints or to alterations on general protein trafficking. Besides, we found that the GPG-motif was also required for Nef-dependent inhibition of ring actin re-organization upon TCR triggering and MHCI downregulation, suggesting that the GPG-motif could actively cooperate with the Nef PxxP motif for these HIV-1 Nef-related effects. Finally, we observed that the Nef-GPG motif was required for optimal infectivity of those viruses produced in T-cells. According to these findings, we propose the conserved GPG-motif in HIV-1 Nef as functional region required for HIV-1 infectivity and therefore with a potential interest for the interference of Nef activity during HIV-1 infection.
Conflict of interest statement
Figures



Similar articles
-
Identification of a highly conserved valine-glycine-phenylalanine amino acid triplet required for HIV-1 Nef function.Retrovirology. 2012 Apr 27;9:34. doi: 10.1186/1742-4690-9-34. Retrovirology. 2012. PMID: 22537596 Free PMC article.
-
An Amino Acid Polymorphism within the HIV-1 Nef Dileucine Motif Functionally Uncouples Cell Surface CD4 and SERINC5 Downregulation.J Virol. 2021 Jul 26;95(16):e0058821. doi: 10.1128/JVI.00588-21. Epub 2021 Jul 26. J Virol. 2021. PMID: 34037423 Free PMC article.
-
Multifunctional Roles of the N-Terminal Region of HIV-1SF2Nef Are Mediated by Three Independent Protein Interaction Sites.J Virol. 2019 Dec 12;94(1):e01398-19. doi: 10.1128/JVI.01398-19. Print 2019 Dec 12. J Virol. 2019. PMID: 31597760 Free PMC article.
-
Two sorting motifs, a ubiquitination motif and a tyrosine motif, are involved in HIV-1 and simian immunodeficiency virus Nef-mediated receptor endocytosis.J Immunol. 2011 May 15;186(10):5807-14. doi: 10.4049/jimmunol.1003506. Epub 2011 Apr 11. J Immunol. 2011. PMID: 21482738
-
Mechanisms of HIV-1 Nef function and intracellular signaling.J Neuroimmune Pharmacol. 2011 Jun;6(2):230-46. doi: 10.1007/s11481-011-9262-y. Epub 2011 Feb 19. J Neuroimmune Pharmacol. 2011. PMID: 21336563 Free PMC article. Review.
Cited by
-
Variation in HIV-1 Nef function within and among viral subtypes reveals genetically separable antagonism of SERINC3 and SERINC5.PLoS Pathog. 2020 Sep 14;16(9):e1008813. doi: 10.1371/journal.ppat.1008813. eCollection 2020 Sep. PLoS Pathog. 2020. PMID: 32925973 Free PMC article.
References
-
- Kestier HW, Ringler DJ, Mori K, Panicali DL, Sehgal PK, Daniel MD, et al. (1991) Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65: 651–662. - PubMed
-
- Baur AS, Sawai ET, Dazin P, Fantl WJ, Cheng-Mayer C, Peterlin BM (1994) HIV-1 Nef leads to inhibition or activation of T cells depending on its intracellular localization. Immunity 1: 373–384. - PubMed
-
- Birch MR, Learmont JC, Dyer WB, Deacon NJ, Zaunders JJ, Saksena N, et al. (2001) An examination of signs of disease progression in survivors of the Sydney Blood Bank Cohort (SBBC). J Clin Virol 22: 263–270. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

