Identification of T-cell Receptors Targeting KRAS-Mutated Human Tumors
- PMID: 26701267
- PMCID: PMC4775432
- DOI: 10.1158/2326-6066.CIR-15-0188
Identification of T-cell Receptors Targeting KRAS-Mutated Human Tumors
Abstract
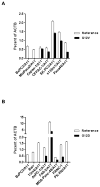
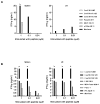
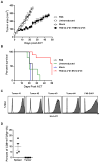
KRAS is one of the most frequently mutated proto-oncogenes in human cancers. The dominant oncogenic mutations of KRAS are single amino acid substitutions at codon 12, in particular G12D and G12V present in 60% to 70% of pancreatic cancers and 20% to 30% of colorectal cancers. The consistency, frequency, and tumor specificity of these "neoantigens" make them attractive therapeutic targets. Recent data associate T cells that target mutated antigens with clinical immunotherapy responses in patients with metastatic melanoma, lung cancer, or cholangiocarcinoma. Using HLA-peptide prediction algorithms, we noted that HLA-A*11:01 could potentially present mutated KRAS variants. By immunizing HLA-A*11:01 transgenic mice, we generated murine T cells and subsequently isolated T-cell receptors (TCR) highly reactive to the mutated KRAS variants G12V and G12D. Peripheral blood lymphocytes (PBL) transduced with these TCRs could recognize multiple HLA-A*11:01(+) tumor lines bearing the appropriate KRAS mutations. In a xenograft model of large established tumor, adoptive transfer of these transduced PBLs reactive with an HLA-A*11:01, G12D-mutated pancreatic cell line could significantly reduce its growth in NSG mice (P = 0.002). The success of adoptive transfer of TCR-engineered T cells against melanoma and other cancers supports clinical trials with these T cells that recognize mutated KRAS in patients with a variety of common cancer types.
©2015 American Association for Cancer Research.
Conflict of interest statement
Figures


References
-
- Schultz NA, Roslind A, Christensen IJ, Horn T, Hogdall E, Pedersen LN, et al. Frequencies and prognostic role of KRAS and BRAF mutations in patients with localized pancreatic and ampullary adenocarcinomas. Pancreas. 2012;41(5):759–66. - PubMed
-
- Hruban RH, van Mansfeld AD, Offerhaus GJ, van Weering DH, Allison DC, Goodman SN, et al. K-ras oncogene activation in adenocarcinoma of the human pancreas. A study of 82 carcinomas using a combination of mutant-enriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. The American journal of pathology. 1993;143(2):545–54. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

