ABHD17 proteins are novel protein depalmitoylases that regulate N-Ras palmitate turnover and subcellular localization
- PMID: 26701913
- PMCID: PMC4755737
- DOI: 10.7554/eLife.11306
ABHD17 proteins are novel protein depalmitoylases that regulate N-Ras palmitate turnover and subcellular localization
Abstract
Dynamic changes in protein S-palmitoylation are critical for regulating protein localization and signaling. Only two enzymes - the acyl-protein thioesterases APT1 and APT2 - are known to catalyze palmitate removal from cytosolic cysteine residues. It is unclear if these enzymes act constitutively on all palmitoylated proteins, or if additional depalmitoylases exist. Using a dual pulse-chase strategy comparing palmitate and protein half-lives, we found knockdown or inhibition of APT1 and APT2 blocked depalmitoylation of Huntingtin, but did not affect palmitate turnover on postsynaptic density protein 95 (PSD95) or N-Ras. We used activity profiling to identify novel serine hydrolase targets of the APT1/2 inhibitor Palmostatin B, and discovered that a family of uncharacterized ABHD17 proteins can accelerate palmitate turnover on PSD95 and N-Ras. ABHD17 catalytic activity is required for N-Ras depalmitoylation and re-localization to internal cellular membranes. Our findings indicate that the family of depalmitoylation enzymes may be substantially broader than previously believed.
Keywords: ABHD17A; ABHD17B; ABHD17C; APT1; Acyl Protein Thioesterase; FAM108A1; FAM108B1; FAM108C1; N-Ras; PSD95; Palmostatin B; biochemistry; cell biology; depalmitoylation; human; palmitoylation.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures

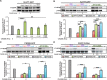
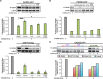

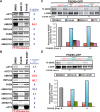
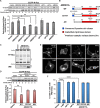
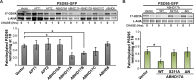
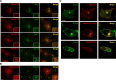
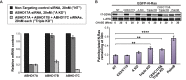

Similar articles
-
Dynamic palmitoylation links cytosol-membrane shuttling of acyl-protein thioesterase-1 and acyl-protein thioesterase-2 with that of proto-oncogene H-ras product and growth-associated protein-43.J Biol Chem. 2013 Mar 29;288(13):9112-25. doi: 10.1074/jbc.M112.421073. Epub 2013 Feb 8. J Biol Chem. 2013. PMID: 23396970 Free PMC article.
-
Identification of PSD-95 Depalmitoylating Enzymes.J Neurosci. 2016 Jun 15;36(24):6431-44. doi: 10.1523/JNEUROSCI.0419-16.2016. J Neurosci. 2016. PMID: 27307232 Free PMC article.
-
Protein depalmitoylases.Crit Rev Biochem Mol Biol. 2018 Feb;53(1):83-98. doi: 10.1080/10409238.2017.1409191. Epub 2017 Dec 14. Crit Rev Biochem Mol Biol. 2018. PMID: 29239216 Free PMC article. Review.
-
Enzymatic protein depalmitoylation by acyl protein thioesterases.Biochem Soc Trans. 2015 Apr;43(2):193-8. doi: 10.1042/BST20140235. Biochem Soc Trans. 2015. PMID: 25849916 Review.
-
Targeting the Ras palmitoylation/depalmitoylation cycle in cancer.Biochem Soc Trans. 2017 Aug 15;45(4):913-921. doi: 10.1042/BST20160303. Epub 2017 Jun 19. Biochem Soc Trans. 2017. PMID: 28630138 Review.
Cited by
-
Trypanosoma brucei Acyl-Protein Thioesterase-like (TbAPT-L) Is a Lipase with Esterase Activity for Short and Medium-Chain Fatty Acids but Has No Depalmitoylation Activity.Pathogens. 2022 Oct 27;11(11):1245. doi: 10.3390/pathogens11111245. Pathogens. 2022. PMID: 36364996 Free PMC article.
-
Protein S-palmitoylation in immunity.Open Biol. 2021 Mar;11(3):200411. doi: 10.1098/rsob.200411. Epub 2021 Mar 3. Open Biol. 2021. PMID: 33653086 Free PMC article. Review.
-
ABHD17 regulation of plasma membrane palmitoylation and N-Ras-dependent cancer growth.Nat Chem Biol. 2021 Aug;17(8):856-864. doi: 10.1038/s41589-021-00785-8. Epub 2021 Apr 29. Nat Chem Biol. 2021. PMID: 33927411 Free PMC article.
-
VPS35 binds farnesylated N-Ras in the cytosol to regulate N-Ras trafficking.J Cell Biol. 2016 Aug 15;214(4):445-58. doi: 10.1083/jcb.201604061. Epub 2016 Aug 8. J Cell Biol. 2016. PMID: 27502489 Free PMC article.
-
APT1-Mediated Depalmitoylation Regulates Hippocampal Synaptic Plasticity.J Neurosci. 2022 Mar 30;42(13):2662-2677. doi: 10.1523/JNEUROSCI.1741-21.2022. Epub 2022 Feb 14. J Neurosci. 2022. PMID: 35165175 Free PMC article.
References
-
- Adibekian A, Martin BR, Chang JW, Hsu K-L, Tsuboi K, Bachovchin DA, Speers AE, Brown SJ, Spicer T, Fernandez-Vega V, Ferguson J, Hodder PS, Rosen H, Cravatt BF. Confirming target engagement for reversible inhibitors in vivo by kinetically tuned activity-based probes. Journal of the American Chemical Society. 2012;134:10345–10348. doi: 10.1021/ja303400u. - DOI - PMC - PubMed
-
- Bachovchin DA, Ji T, Li W, Simon GM, Blankman JL, Adibekian A, Hoover H, Niessen S, Cravatt BF. Superfamily-wide portrait of serine hydrolase inhibition achieved by library-versus-library screening. Proceedings of the National Academy of Sciences. 2010;107:20941–20946. doi: 10.1073/pnas.1011663107. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

