Severity and Frequency of Proximal Tubule Injury Determines Renal Prognosis
- PMID: 26701981
- PMCID: PMC4978049
- DOI: 10.1681/ASN.2015060647
Severity and Frequency of Proximal Tubule Injury Determines Renal Prognosis
Abstract
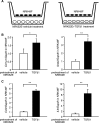
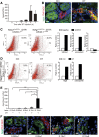
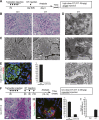
AKI increases the risk of developing CKD, but the mechanisms linking AKI to CKD remain unclear. Because proximal tubule injury is the mainstay of AKI, we postulated that proximal tubule injury triggers features of CKD. We generated a novel mouse model to induce proximal tubule-specific adjustable injury by inducing the expression of diphtheria toxin (DT) receptor with variable prevalence in proximal tubules. Administration of high-dose DT in mice expressing the DT receptor consistently caused severe proximal tubule-specific injury associated with interstitial fibrosis and reduction of erythropoietin production. Mild proximal tubule injury from a single injection of low-dose DT triggered reversible fibrosis, whereas repeated mild injuries caused sustained interstitial fibrosis, inflammation, glomerulosclerosis, and atubular glomeruli. DT-induced proximal tubule-specific injury also triggered distal tubule injury. Furthermore, injured tubular cells cocultured with fibroblasts stimulated induction of extracellular matrix and inflammatory genes. These results support the existence of proximal-distal tubule crosstalk and crosstalk between tubular cells and fibroblasts. Overall, our data provide evidence that proximal tubule injury triggers several features of CKD and that the severity and frequency of proximal tubule injury determines the progression to CKD.
Keywords: acute renal failure; chronic kidney disease; proximal tubule.
Copyright © 2016 by the American Society of Nephrology.
Figures
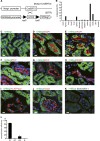
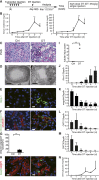
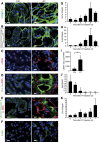
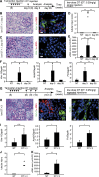

References
-
- Thadhani R, Pascual M, Bonventre JV: Acute renal failure. N Engl J Med 334: 1448–1460, 1996 - PubMed
-
- Hsu CY: Where is the epidemic in kidney disease? J Am Soc Nephrol 21: 1607–1611, 2010 - PubMed
-
- Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 - PubMed
-
- Leung KC, Tonelli M, James MT: Chronic kidney disease following acute kidney injury-risk and outcomes. Nat Rev Nephrol 9: 77–85, 2013 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

