Heat acclimation and thirst in rats
- PMID: 26702076
- PMCID: PMC4760436
- DOI: 10.14814/phy2.12642
Heat acclimation and thirst in rats
Abstract
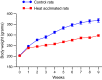
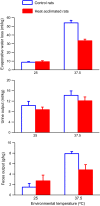
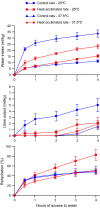
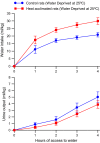
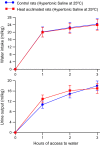
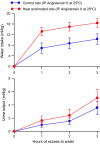
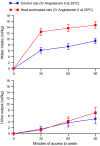
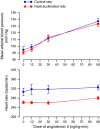
The effects of heat acclimation on water intake and urine output responses to thermal dehydration and other thirst stimuli were studied in male Sprague-Dawley rats. Rats were heat acclimated by continuous exposure to a 34°C environment for at least 6 weeks. Thermal dehydration-induced thirst was brought about by exposing the heat-acclimated rats and control rats housed at 24°C to a 37.5°C environment for 4 h without access to food or water. Heat acclimation reduced evaporative and urinary water losses and the increases in plasma sodium and osmolality during thermal dehydration, which led to a reduction in thermal dehydration-induced thirst. Heat acclimation reduced the rate of rehydration following thermal dehydration but did not alter the final rehydration level, indicating that heat acclimation does not alter the primary control of thermal dehydration-induced thirst. Heat acclimation did not alter water intake or urine output following administration of hypertonic saline, which selectively stimulates intracellular thirst, but led to greater water intake following administration of angiotensin II, which plays an important role in extracellular/volemic thirst, and following water deprivation, which activates both thirst pathways. Cardiovascular responses to angiotensin II were not altered by heat acclimation. Heat acclimation thus reduces water loss during heat exposure in rats, but does not have major effects on thermal dehydration-induced or extracellular thirst but does appear to alter volemic thirst.
Keywords: Angiotensin II; intracellular thirst; kidney; rehydration; thermal dehydration; volemic thirst.
© 2015 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of the American Physiological Society and The Physiological Society.
Figures
References
-
- Arieli, A. , and Chinet A.. 1986. Thyroid status and noradrenaline‐induced regulatory thermogenesis in heat acclimated rats. Horm. Metab. Res. 18:103–106. - PubMed
-
- Attah, M. Y. , and Besch E. L.. 1977. Estrous cycle variations of food and water intake in rats in the heat. J. Appl. Physiol. 42:874–877. - PubMed
-
- Banji, D. , Banji O. J. F., Pavani B., Kumar C. K., and Annamalai A. R.. 2014. Zingerone regulates intestinal transit, attenuates behavioral and oxidative perturbations in irritable bowel disorder in rats. Phytomedicine 21:423–429. - PubMed
-
- Barney, C. C. 1997. Effects of preloads of water and saline on thermal dehydration‐induced thirst. Physiol. Behav. 61:763–769. - PubMed
-
- Barney, C. C. , and Folkerts M. M.. 1995. Thermal dehydration‐induced thirst in rats: role of body temperature. Am. J. Physiol. 269:R557–R564. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

