Increased peripheral vascular disease risk progressively constrains perfusion adaptability in the skeletal muscle microcirculation
- PMID: 26702145
- PMCID: PMC4796615
- DOI: 10.1152/ajpheart.00790.2015
Increased peripheral vascular disease risk progressively constrains perfusion adaptability in the skeletal muscle microcirculation
Abstract
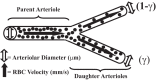
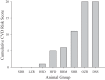
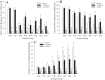
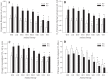
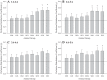
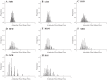
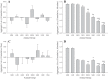
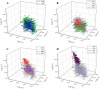
To determine the impact of progressive elevations in peripheral vascular disease (PVD) risk on microvascular function, we utilized eight rat models spanning "healthy" to "high PVD risk" and used a multiscale approach to interrogate microvascular function and outcomes: healthy: Sprague-Dawley rats (SDR) and lean Zucker rats (LZR); mild risk: SDR on high-salt diet (HSD) and SDR on high-fructose diet (HFD); moderate risk: reduced renal mass-hypertensive rats (RRM) and spontaneously hypertensive rats (SHR); high risk: obese Zucker rats (OZR) and Dahl salt-sensitive rats (DSS). Vascular reactivity and biochemical analyses demonstrated that even mild elevations in PVD risk severely attenuated nitric oxide (NO) bioavailability and caused progressive shifts in arachidonic acid metabolism, increasing thromboxane A2 levels. With the introduction of hypertension, arteriolar myogenic activation and adrenergic constriction were increased. However, while functional hyperemia and fatigue resistance of in situ skeletal muscle were not impacted with mild or moderate PVD risk, blood oxygen handling suggested an increasingly heterogeneous perfusion within resting and contracting skeletal muscle. Analysis of in situ networks demonstrated an increasingly stable and heterogeneous distribution of perfusion at arteriolar bifurcations with elevated PVD risk, a phenomenon that was manifested first in the distal microcirculation and evolved proximally with increasing risk. The increased perfusion distribution heterogeneity and loss of flexibility throughout the microvascular network, the result of the combined effects on NO bioavailability, arachidonic acid metabolism, myogenic activation, and adrenergic constriction, may represent the most accurate predictor of the skeletal muscle microvasculopathy and poor health outcomes associated with chronic elevations in PVD risk.
Keywords: blood flow regulation; microvascular dysfunction; peripheral vascular disease; rodent models of cardiovascular disease risk; system biology of microcirculation.
Copyright © 2016 the American Physiological Society.
Figures


References
-
- Aleixandre de Artiñano A, Miguel Castro M. Experimental rat models to study the metabolic syndrome. Br J Nutr 102: 1246–1253, 2009. - PubMed
-
- Baker M, Wayland H. On-line volume-flow rates and velocity profile measurements for blood in microvessels. Microvasc Res 7: 131–143, 1974. - PubMed
-
- Boudreau DM, Malone DC, Raebel MA, Fishman PA, Nichols GA, Feldstein AC, Boscoe AN, Ben-Joseph RH, Magid DJ, Okamoto LJ. Health care utilization and costs by metabolic syndrome risk factors. Metab Syndr Relat Disord 7: 305–314, 2009. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

