A System Dynamics Model of Serum Prostate-Specific Antigen Screening for Prostate Cancer
- PMID: 26702631
- PMCID: PMC4724096
- DOI: 10.1093/aje/kwv262
A System Dynamics Model of Serum Prostate-Specific Antigen Screening for Prostate Cancer
Erratum in
-
Re: "A System Dynamics Model of Serum Prostate-Specific Antigen Screening for Prostate Cancer".Am J Epidemiol. 2016 Mar 15;183(6):597. doi: 10.1093/aje/kww032. Am J Epidemiol. 2016. PMID: 26957009 Free PMC article. No abstract available.
Abstract
Since 2012, US guidelines have recommended against prostate-specific antigen (PSA) screening for prostate cancer. However, evidence of screening benefit from the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening trial and the European Randomized Study of Screening for Prostate Cancer has been inconsistent, due partly to differences in noncompliance and contamination. Using system dynamics modeling, we replicated the PLCO trial and extrapolated follow-up to 20 years. We then simulated 3 scenarios correcting for contamination in the PLCO control arm using Surveillance, Epidemiology, and End Results (SEER) incidence and survival data collected prior to the PSA screening era (scenario 1), SEER data collected during the PLCO trial period (1993-2001) (scenario 2), and data from the European trial's control arm (1991-2005) (scenario 3). In all scenarios, noncompliance was corrected using incidence and survival rates for men with screen-detected cancer in the PLCO screening arm. Scenarios 1 and 3 showed a benefit of PSA screening, with relative risks of 0.62 (95% confidence interval: 0.53, 0.72) and 0.70 (95% confidence interval: 0.59, 0.83) for cancer-specific mortality after 20 years, respectively. In scenario 2, however, there was no benefit of screening. This simulation showed that after correcting for noncompliance and contamination, there is potential benefit of PSA screening in reducing prostate cancer mortality. It also demonstrates the utility of system dynamics modeling for synthesizing epidemiologic evidence to inform public policy.
Keywords: cancer-specific mortality; policy evaluation; prostate cancer; prostate-specific antigen screening; system dynamics modeling.
© The Author 2015. Published by Oxford University Press on behalf of the Johns Hopkins Bloomberg School of Public Health. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
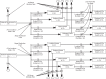
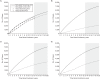
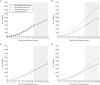
References
-
- American Cancer Society. Cancer Facts and Figures: 2009. Atlanta, GA: American Cancer Society; 2009.
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;621:10–29. - PubMed
-
- Moyer VA; U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;1572:120–134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

