Patch-based augmentation of Expectation-Maximization for brain MRI tissue segmentation at arbitrary age after premature birth
- PMID: 26702777
- PMCID: PMC4755845
- DOI: 10.1016/j.neuroimage.2015.12.009
Patch-based augmentation of Expectation-Maximization for brain MRI tissue segmentation at arbitrary age after premature birth
Abstract
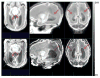
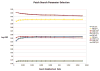
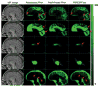
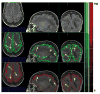
Accurate automated tissue segmentation of premature neonatal magnetic resonance images is a crucial task for quantification of brain injury and its impact on early postnatal growth and later cognitive development. In such studies it is common for scans to be acquired shortly after birth or later during the hospital stay and therefore occur at arbitrary gestational ages during a period of rapid developmental change. It is important to be able to segment any of these scans with comparable accuracy. Previous work on brain tissue segmentation in premature neonates has focused on segmentation at specific ages. Here we look at solving the more general problem using adaptations of age specific atlas based methods and evaluate this using a unique manually traced database of high resolution images spanning 20 gestational weeks of development. We examine the complimentary strengths of age specific atlas-based Expectation-Maximization approaches and patch-based methods for this problem and explore the development of two new hybrid techniques, patch-based augmentation of Expectation-Maximization with weighted fusion and a spatial variability constrained patch search. The former approach seeks to combine the advantages of both atlas- and patch-based methods by learning from the performance of the two techniques across the brain anatomy at different developmental ages, while the latter technique aims to use anatomical variability maps learnt from atlas training data to locally constrain the patch-based search range. The proposed approaches were evaluated using leave-one-out cross-validation. Compared with the conventional age specific atlas-based segmentation and direct patch based segmentation, both new approaches demonstrate improved accuracy in the automated labeling of cortical gray matter, white matter, ventricles and sulcal cortical-spinal fluid regions, while maintaining comparable results in deep gray matter.
Keywords: Atlas-based; Expectation–Maximization; MRI; Patch-based; Premature neonates; Segmentation; Spatio-temporal.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures



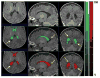
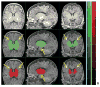
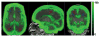


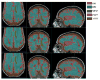
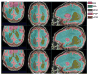
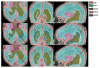
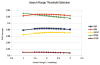
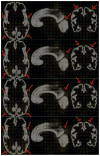
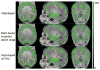
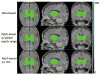
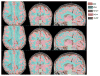

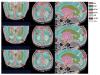
Similar articles
-
Segmentation of neonatal brain MR images using patch-driven level sets.Neuroimage. 2014 Jan 1;84:141-58. doi: 10.1016/j.neuroimage.2013.08.008. Epub 2013 Aug 19. Neuroimage. 2014. PMID: 23968736 Free PMC article.
-
Subject-specific atlas for automatic brain tissue segmentation of neonatal magnetic resonance images.Sci Rep. 2024 Aug 18;14(1):19114. doi: 10.1038/s41598-024-69995-z. Sci Rep. 2024. PMID: 39155321 Free PMC article.
-
Automatic segmentation of eight tissue classes in neonatal brain MRI.PLoS One. 2013 Dec 17;8(12):e81895. doi: 10.1371/journal.pone.0081895. eCollection 2013. PLoS One. 2013. PMID: 24358132 Free PMC article.
-
Neonatal brain MRI segmentation: A review.Comput Biol Med. 2015 Sep;64:163-78. doi: 10.1016/j.compbiomed.2015.06.016. Epub 2015 Jun 29. Comput Biol Med. 2015. PMID: 26189155 Review.
-
Quantifying deep grey matter atrophy using automated segmentation approaches: A systematic review of structural MRI studies.Neuroimage. 2019 Nov 1;201:116018. doi: 10.1016/j.neuroimage.2019.116018. Epub 2019 Jul 15. Neuroimage. 2019. PMID: 31319182
Cited by
-
Postnatal polyunsaturated fatty acids associated with larger preterm brain tissue volumes and better outcomes.Pediatr Res. 2018 Jan;83(1-1):93-101. doi: 10.1038/pr.2017.230. Epub 2017 Oct 18. Pediatr Res. 2018. PMID: 28915231 Free PMC article.
-
NEOCIVET: Towards accurate morphometry of neonatal gyrification and clinical applications in preterm newborns.Neuroimage. 2016 Sep;138:28-42. doi: 10.1016/j.neuroimage.2016.05.034. Epub 2016 May 13. Neuroimage. 2016. PMID: 27184202 Free PMC article.
-
Role of deep learning in infant brain MRI analysis.Magn Reson Imaging. 2019 Dec;64:171-189. doi: 10.1016/j.mri.2019.06.009. Epub 2019 Jun 20. Magn Reson Imaging. 2019. PMID: 31229667 Free PMC article. Review.
-
Baby brain atlases.Neuroimage. 2019 Jan 15;185:865-880. doi: 10.1016/j.neuroimage.2018.04.003. Epub 2018 Apr 3. Neuroimage. 2019. PMID: 29625234 Free PMC article. Review.
-
Learning Cortical Parcellations Using Graph Neural Networks.Front Neurosci. 2021 Dec 24;15:797500. doi: 10.3389/fnins.2021.797500. eCollection 2021. Front Neurosci. 2021. PMID: 35002611 Free PMC article.
References
-
- Aljabar P, Heckemann RA, Hammers A, Hajnal JV, Rueckert D. Multi-atlas based segmentation of brain images: atlas selection and its effect on accuracy. NeuroImage. 2009;46:726–738. - PubMed
-
- Artaechevarria X, Munoz-Barrutia A, Ortizde Solórzano C. Combination strategies in multi-atlas image segmentation: Application to brain mr data. Medical Imaging, IEEE Transactions on. 2009;28:1266–1277. - PubMed
-
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–851. http://dx.doi.org/10.1016/j.neuroimage.2005.02.018. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

