Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study
- PMID: 26703889
- PMCID: PMC6063317
- DOI: 10.1016/S0140-6736(15)00817-X
Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study
Abstract
Background: Effective systemic therapies for patients with advanced, progressive neuroendocrine tumours of the lung or gastrointestinal tract are scarce. We aimed to assess the efficacy and safety of everolimus compared with placebo in this patient population.
Methods: In the randomised, double-blind, placebo-controlled, phase 3 RADIANT-4 trial, adult patients (aged ≥18 years) with advanced, progressive, well-differentiated, non-functional neuroendocrine tumours of lung or gastrointestinal origin were enrolled from 97 centres in 25 countries worldwide. Eligible patients were randomly assigned in a 2:1 ratio by an interactive voice response system to receive everolimus 10 mg per day orally or identical placebo, both with supportive care. Patients were stratified by tumour origin, performance status, and previous somatostatin analogue treatment. Patients, investigators, and the study sponsor were masked to treatment assignment. The primary endpoint was progression-free survival assessed by central radiology review, analysed by intention to treat. Overall survival was a key secondary endpoint. This trial is registered with ClinicalTrials.gov, number NCT01524783.
Findings: Between April 3, 2012, and Aug 23, 2013, a total of 302 patients were enrolled, of whom 205 were allocated to everolimus 10 mg per day and 97 to placebo. Median progression-free survival was 11·0 months (95% CI 9·2-13·3) in the everolimus group and 3·9 months (3·6-7·4) in the placebo group. Everolimus was associated with a 52% reduction in the estimated risk of progression or death (hazard ratio [HR] 0·48 [95% CI 0·35-0·67], p<0·00001). Although not statistically significant, the results of the first pre-planned interim overall survival analysis indicated that everolimus might be associated with a reduction in the risk of death (HR 0·64 [95% CI 0·40-1·05], one-sided p=0·037, whereas the boundary for statistical significance was 0·0002). Grade 3 or 4 drug-related adverse events were infrequent and included stomatitis (in 18 [9%] of 202 patients in the everolimus group vs 0 of 98 in the placebo group), diarrhoea (15 [7%] vs 2 [2%]), infections (14 [7%] vs 0), anaemia (8 [4%] vs 1 [1%]), fatigue (7 [3%] vs 1 [1%]), and hyperglycaemia (7 [3%] vs 0).
Interpretation: Treatment with everolimus was associated with significant improvement in progression-free survival in patients with progressive lung or gastrointestinal neuroendocrine tumours. The safety findings were consistent with the known side-effect profile of everolimus. Everolimus is the first targeted agent to show robust anti-tumour activity with acceptable tolerability across a broad range of neuroendocrine tumours, including those arising from the pancreas, lung, and gastrointestinal tract.
Funding: Novartis Pharmaceuticals Corporation.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
JCY has received consulting or advisory fees from Ipsen, Lexicon, and Novartis and research funding from Novartis. NF has received honoraria from Ipsen, and Novartis, consulting or advisory fees from Ipsen, Lexicon, Novartis, and Italfarmaco, research funding from Novartis, and travel and accommodations expenses from Ipsen and Novartis. SS has received honoraria, consulting or advisory fees, travel and accommodations expenses, and research funding from Novartis. RB has received research funding from Italfarmaco, Novartis, and Otsuka and travel and accommodations expenses from Ipsen; Italfarmaco; and Novartis. EMW has received consulting or advisory fees from Celgene, Ipsen, and Novartis. JT has received honoraria, research funding, and travel and accommodations expenses from Novartis. MR has received honoraria from Celgene, Ipsen, Novartis, and Roche and consulting or advisory fees from Celgene, Ipsen, Novartis, and Roche. HL has received honoraria from Ipsen, Novartis, and Pfizer, consulting or advisory fees from Novartis and Pfizer, research funding from Novartis and travel and accommodations expenses from Ipsen, Novartis, and Pfizer. MV, LBP, NR, and CS are employees of and own shares in Novartis. JWV has received honoraria, consulting or advisory fees and research funding from Novartis. GDF has received consulting or advisory fees and research funding from Novartis. EVC has received research funding from Novartis. YS has received research funding Chugai Pharma, Lilly, Novartis, and Taiho Pharmaceutical. JS has received honoraria from Novartis, consulting or advisory fees from Ipsen, Lexicon, and Novartis, research funding from Novartis and Pfizer, and is on the speaker’s bureau for Bayer and Genentech. MHK has received consulting or advisory fees from Ipsen and Novartis. MEP has received honoraria from Ipsen, Lexicon, Novartis, and Pfizer, consulting or advisory fees from Ipsen, Lexicon, Novartis, and Pfizer, research funding from Novartis, and travel and accommodations expenses from Ipsen and Novartis. CC, MT, and DYO declare no conflicts of interest.
Figures







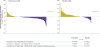
Comment in
-
Universal everolimus for malignant neuroendocrine tumours?Lancet. 2016 Mar 5;387(10022):924-926. doi: 10.1016/S0140-6736(15)01234-9. Epub 2015 Dec 17. Lancet. 2016. PMID: 26703890 No abstract available.
-
Everolimus in ileum neuroendocrine tumours.Lancet. 2016 Jul 16;388(10041):236. doi: 10.1016/S0140-6736(16)31039-X. Lancet. 2016. PMID: 27479567 No abstract available.
-
Everolimus in ileum neuroendocrine tumours - Authors' reply.Lancet. 2016 Jul 16;388(10041):236-7. doi: 10.1016/S0140-6736(16)31045-5. Lancet. 2016. PMID: 27479568 No abstract available.
References
-
- Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–72. - PubMed
-
- Choti MA, Bobiak S, Strosberg JR, et al. Prevalence of functional tumors in neuroendocrine carcinoma: An analysis from the NCCN NET database. J Clin Oncol. 2012;30:4126. (abstr).
-
- Yao J, Phan A. Optimising therapeutic options for patients with advanced pancreatic neuroendocrine tumours. Eur Oncol Haematol. 2012;8:217–23.
-
- Rinke A, Muller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656–63. - PubMed
-
- Caplin ME, Pavel M, Ruszniewski P. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224–33. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

