Supplemental parenteral nutrition in critically ill patients: a study protocol for a phase II randomised controlled trial
- PMID: 26703919
- PMCID: PMC4690293
- DOI: 10.1186/s13063-015-1118-y
Supplemental parenteral nutrition in critically ill patients: a study protocol for a phase II randomised controlled trial
Abstract
Background: Nutrition is one of the fundamentals of care provided to critically ill adults. The volume of enteral nutrition received, however, is often much less than prescribed due to multiple functional and process issues. To deliver the prescribed volume and correct the energy deficit associated with enteral nutrition alone, parenteral nutrition can be used in combination (termed "supplemental parenteral nutrition"), but benefits of this method have not been firmly established. A multi-centre, randomised, clinical trial is currently underway to determine if prescribed energy requirements can be provided to critically ill patients by using a supplemental parenteral nutrition strategy in the critically ill.
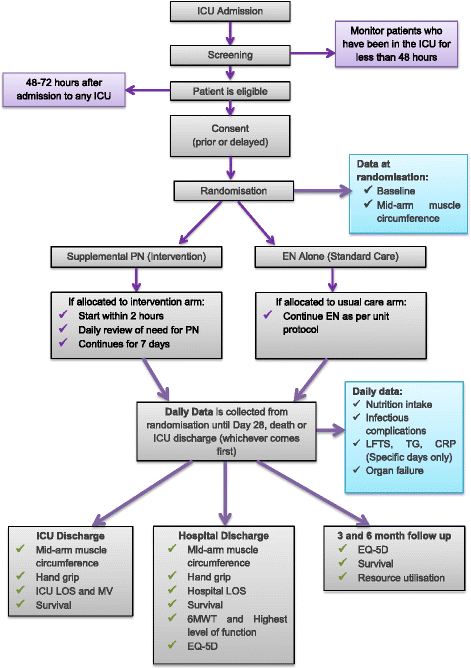
Methods/design: This prospective, multi-centre, randomised, stratified, parallel-group, controlled, phase II trial aims to determine whether a supplemental parenteral nutrition strategy will reliably and safely increase energy intake when compared to usual care. The study will be conducted for 100 critically ill adults with at least one organ system failure and evidence of insufficient enteral intake from six intensive care units in Australia and New Zealand. Enrolled patients will be allocated to either a supplemental parenteral nutrition strategy for 7 days post randomisation or to usual care with enteral nutrition. The primary outcome will be the average energy amount delivered from nutrition therapy over the first 7 days of the study period. Secondary outcomes include protein delivery for 7 days post randomisation; total energy and protein delivery, antibiotic use and organ failure rates (up to 28 days); duration of ventilation, length of intensive care unit and hospital stay. At both intensive care unit and hospital discharge strength and health-related quality of life assessments will be undertaken. Study participants will be followed up for health-related quality of life, resource utilisation and survival at 90 and 180 days post randomisation (unless death occurs first).
Discussion: This trial aims to determine if provision of a supplemental parenteral nutrition strategy to critically ill adults will increase energy intake compared to usual care in Australia and New Zealand. Trial outcomes will guide development of a subsequent larger randomised controlled trial.
Trial registration: NCT01847534 (First registered 5 February 2013, last updated 14 October 2015).
Figures

References
-
- Martindale RG, McClave SA, Vanek VW, McCarthy M, Roberts P, Taylor B, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition: Executive Summary. Crit Care Med. 2009;37(5):1757–61. doi: 10.1097/CCM.0b013e3181a40116. - DOI - PubMed
-
- Doig GS, Heighes PT, Simpson F, Sweetman EA, Davies AR. Early enteral nutrition, provided within 24 h of injury or intensive care unit admission, significantly reduces mortality in critically ill patients: a meta-analysis of randomised controlled trials. Intensive Care Med. 2009;35(12):2018–27. doi: 10.1007/s00134-009-1664-4. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

