Response to treatment in a prospective national infantile spasms cohort
- PMID: 26704170
- PMCID: PMC5902168
- DOI: 10.1002/ana.24594
Response to treatment in a prospective national infantile spasms cohort
Abstract
Objective: Infantile spasms are seizures associated with a severe epileptic encephalopathy presenting in the first 2 years of life, and optimal treatment continues to be debated. This study evaluates early and sustained response to initial treatments and addresses both clinical remission and electrographic resolution of hypsarrhythmia. Secondarily, it assesses whether response to treatment differs by etiology or developmental status.
Methods: The National Infantile Spasms Consortium established a multicenter, prospective database enrolling infants with new diagnosis of infantile spasms. Children were considered responders if there was clinical remission and resolution of hypsarrhythmia that was sustained at 3 months after first treatment initiation. Standard treatments of adrenocorticotropic hormone (ACTH), oral corticosteroids, and vigabatrin were considered individually, and all other nonstandard therapies were analyzed collectively. Developmental status and etiology were assessed. We compared response rates by treatment group using chi-square tests and multivariate logistic regression models.
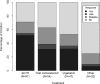
Results: Two hundred thirty infants were enrolled from 22 centers. Overall, 46% of children receiving standard therapy responded, compared to only 9% who responded to nonstandard therapy (p < 0.001). Fifty-five percent of infants receiving ACTH as initial treatment responded, compared to 39% for oral corticosteroids, 36% for vigabatrin, and 9% for other (p < 0.001). Neither etiology nor development significantly modified the response pattern by treatment group.
Interpretation: Response rate varies by treatment choice. Standard therapies should be considered as initial treatment for infantile spasms, including those with impaired development or known structural or genetic/metabolic etiology. ACTH appeared to be more effective than other standard therapies.
© 2016 American Neurological Association.
Conflict of interest statement
Figures
References
-
- Riikonen R, Donner M. Incidence and aetiology of infantile spasms from 1960 to 1976: a population study in Finland. Dev Med Child Neurol. 1979;21:333–343. - PubMed
-
- Cowan LD, Hudson LS. The epidemiology and natural history of infantile spasms. J Child Neurol. 1991;6:355–364. - PubMed
-
- Luthvigsson P, Olafsson E, Sigurthardottir S, Hauser WA. Epidemiologic features of infantile spasms in Iceland. Epilepsia. 1994;35:802–805. - PubMed
-
- Sidenvall R, Eeg-Olofsson O. Epidemiology of infantile spasms in Sweden. Epilepsia. 1995;36:572–574. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical