Tooth and scale morphogenesis in shark: an alternative process to the mammalian enamel knot system
- PMID: 26704180
- PMCID: PMC4690397
- DOI: 10.1186/s12862-015-0557-0
Tooth and scale morphogenesis in shark: an alternative process to the mammalian enamel knot system
Abstract
Background: The gene regulatory network involved in tooth morphogenesis has been extremely well described in mammals and its modeling has allowed predictions of variations in regulatory pathway that may have led to evolution of tooth shapes. However, very little is known outside of mammals to understand how this regulatory framework may also account for tooth shape evolution at the level of gnathostomes. In this work, we describe expression patterns and proliferation/apoptosis assays to uncover homologous regulatory pathways in the catshark Scyliorhinus canicula.
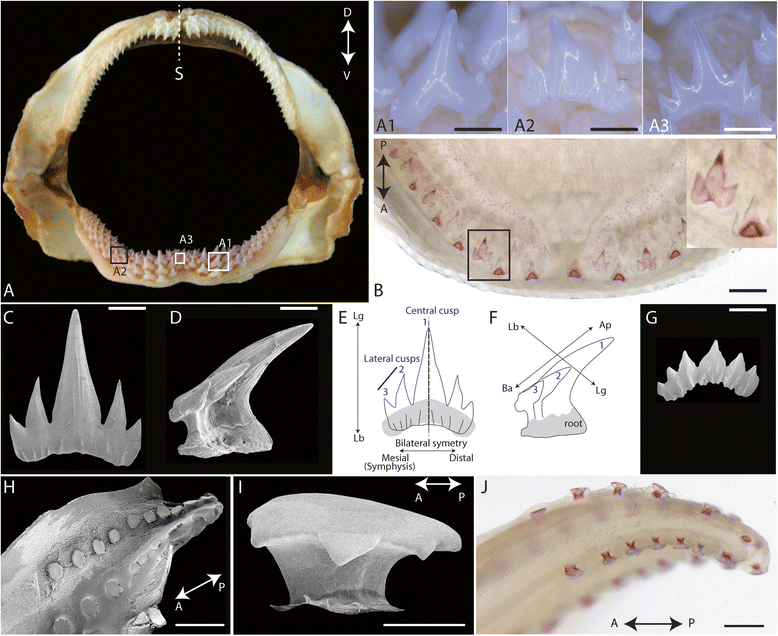
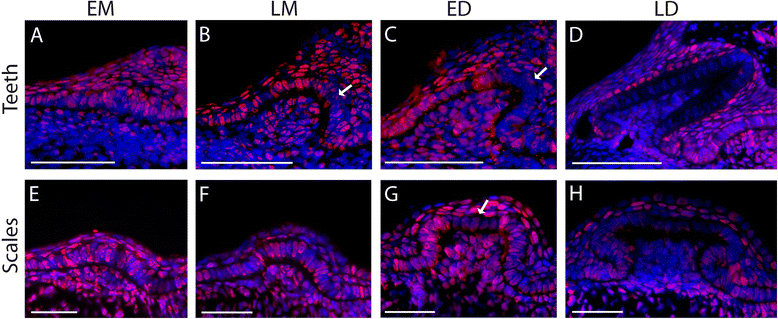
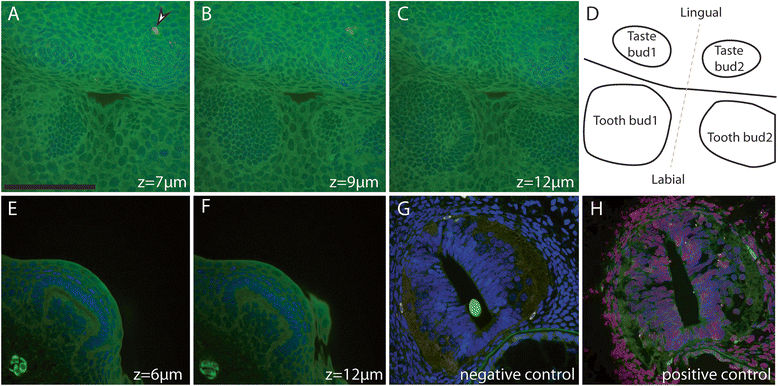
Results: Because of their similar structural and developmental features, gene expression patterns were described over the four developmental stages of both tooth and scale buds in the catshark. These gene expression patterns differ from mouse tooth development, and discrepancies are also observed between tooth and scale development within the catshark. However, a similar nested expression of Shh and Fgf suggests similar signaling involved in morphogenesis of all structures, although apoptosis assays do not support a strictly equivalent enamel knot system in sharks. Similarities in the topology of gene expression pattern, including Bmp signaling pathway, suggest that mouse molar development is more similar to scale bud development in the catshark.
Conclusions: These results support the fact that no enamel knot, as described in mammalian teeth, can be described in the morphogenesis of shark teeth or scales. However, homologous signaling pathways are involved in growth and morphogenesis with variations in their respective expression patterns. We speculate that variations in this topology of expression are also a substrate for tooth shape evolution, notably in regulating the growth axis and symmetry of the developing structure.
Figures






Similar articles
-
An epithelial signalling centre in sharks supports homology of tooth morphogenesis in vertebrates.Elife. 2022 May 10;11:e73173. doi: 10.7554/eLife.73173. Elife. 2022. PMID: 35536602 Free PMC article.
-
Associations of FGF-3 and FGF-10 with signaling networks regulating tooth morphogenesis.Dev Dyn. 2000 Nov;219(3):322-32. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1062>3.0.CO;2-J. Dev Dyn. 2000. PMID: 11066089
-
The life history of an embryonic signaling center: BMP-4 induces p21 and is associated with apoptosis in the mouse tooth enamel knot.Development. 1998 Jan;125(2):161-9. doi: 10.1242/dev.125.2.161. Development. 1998. PMID: 9486790
-
Reiterative signaling and patterning during mammalian tooth morphogenesis.Mech Dev. 2000 Mar 15;92(1):19-29. doi: 10.1016/s0925-4773(99)00322-6. Mech Dev. 2000. PMID: 10704885 Review.
-
Enamel knots as signaling centers linking tooth morphogenesis and odontoblast differentiation.Adv Dent Res. 2001 Aug;15:14-8. doi: 10.1177/08959374010150010401. Adv Dent Res. 2001. PMID: 12640732 Review.
Cited by
-
Interference with the retinoic acid signalling pathway inhibits the initiation of teeth and caudal primary scales in the small-spotted catshark Scyliorhinus canicula.PeerJ. 2023 Sep 6;11:e15896. doi: 10.7717/peerj.15896. eCollection 2023. PeerJ. 2023. PMID: 37692112 Free PMC article.
-
Developmental basis of natural tooth shape variation in cichlid fishes.Naturwissenschaften. 2025 Jan 27;112(1):12. doi: 10.1007/s00114-025-01964-6. Naturwissenschaften. 2025. PMID: 39869142 Free PMC article.
-
An epithelial signalling centre in sharks supports homology of tooth morphogenesis in vertebrates.Elife. 2022 May 10;11:e73173. doi: 10.7554/eLife.73173. Elife. 2022. PMID: 35536602 Free PMC article.
-
Acanthodian dental development and the origin of gnathostome dentitions.Nat Ecol Evol. 2021 Jul;5(7):919-926. doi: 10.1038/s41559-021-01458-4. Epub 2021 May 6. Nat Ecol Evol. 2021. PMID: 33958756
-
Redeployment of odontode gene regulatory network underlies dermal denticle formation and evolution in suckermouth armored catfish.Sci Rep. 2022 Apr 13;12(1):6172. doi: 10.1038/s41598-022-10222-y. Sci Rep. 2022. PMID: 35418659 Free PMC article.
References
-
- Debiais-Thibaud M, Borday-Birraux V, Germon I, Bourrat F, Metcalfe CJ, Casane D, et al. Development of oral and pharyngeal teeth in the medaka (Oryzias latipes): comparison of morphology and expression of eve1 gene. J Exp Zool B Mol Dev Evol. 2007;708(May):693–708. doi: 10.1002/jez.b.21183. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

