Role of HLA-G and extracellular vesicles in renal cancer stem cell-induced inhibition of dendritic cell differentiation
- PMID: 26704308
- PMCID: PMC4690241
- DOI: 10.1186/s12885-015-2025-z
Role of HLA-G and extracellular vesicles in renal cancer stem cell-induced inhibition of dendritic cell differentiation
Abstract
Background: Tumor immune-escape has been related to the ability of cancer cells to inhibit T cell activation and dendritic cell (DC) differentiation. We previously identified a tumor initiating population, expressing the mesenchymal marker CD105, which fulfills the criteria for definition as cancer stem cells (CD105(+) CSCs) able to release extracellular vesicles (EVs) that favor tumor progression and metastases. The aim of the present study was to compare the ability of renal CSCs and derived EVs to modulate the behavior of monocyte-derived DCs with a non-tumor initiating renal cancer cell population (CD105(-) TCs) and their EVs.
Methods: Maturation of monocyte-derived DCs was studied in presence of CD105(+) CSCs and CD105(-) TCs and their derived EVs. DC differentiation experiments were evaluated by cytofluorimetric analysis. T cell proliferation and ELISA assays were performed. Monocytes and T cells were purified from peripheral blood mononuclear cells obtained from healthy donors.
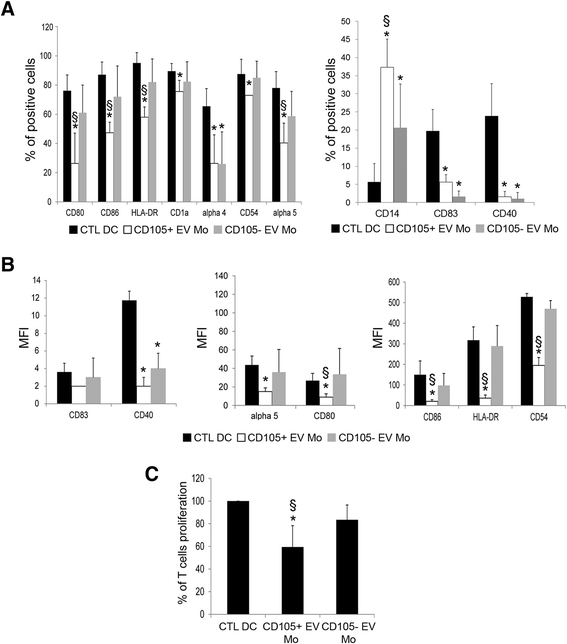
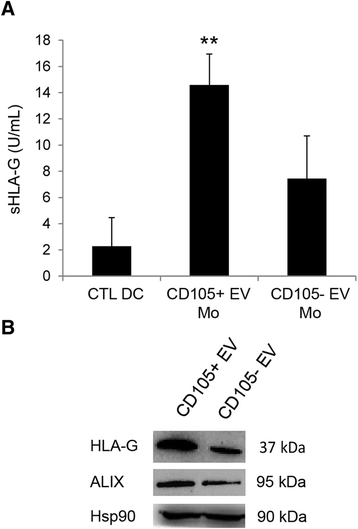
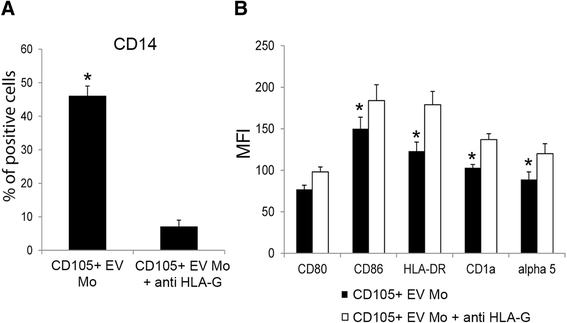
Results: The results obtained demonstrate that both CD105(+) CSCs and CD105(-) TCs impaired the differentiation process of DCs from monocytes. However, the immune-modulatory effect of CD105(+) CSCs was significantly greater than that of CD105(-) TCs. EVs derived from CD105(+) CSCs and in less extent, those derived from CD105(-) TCs retained the ability to impair monocyte maturation and T cell activation. The mechanism has been mainly related to the expression of HLA-G by tumor cells and to its release in a form associated to EVs. HLA-G blockade significantly reduced the inhibitory effect of EVs on DC differentiation.
Conclusions: In conclusion, the results of the present study indicate that renal cancer cells and in particular CSCs and derived EVs impair maturation of DCs and T cell immune response by a mechanism involving HLA-G.
Figures



Similar articles
-
Extracellular vesicles in onco-nephrology.Exp Mol Med. 2019 Mar 15;51(3):1-8. doi: 10.1038/s12276-019-0213-7. Exp Mol Med. 2019. PMID: 30872568 Free PMC article. Review.
-
Human mesenchymal stem cells and derived extracellular vesicles induce regulatory dendritic cells in type 1 diabetic patients.Diabetologia. 2016 Feb;59(2):325-33. doi: 10.1007/s00125-015-3808-0. Epub 2015 Nov 23. Diabetologia. 2016. PMID: 26592240
-
Mesenchymal Stromal Cell-Derived Extracellular Vesicles Attenuate Dendritic Cell Maturation and Function.Front Immunol. 2018 Nov 9;9:2538. doi: 10.3389/fimmu.2018.02538. eCollection 2018. Front Immunol. 2018. PMID: 30473695 Free PMC article.
-
Dendritic-cell-based immunotherapy evokes potent anti-tumor immune responses in CD105+ human renal cancer stem cells.Mol Carcinog. 2017 Nov;56(11):2499-2511. doi: 10.1002/mc.22697. Epub 2017 Sep 14. Mol Carcinog. 2017. PMID: 28621442
-
Diverse functional activity of CD83+ monocyte-derived dendritic cells and the implications for cancer vaccines.Crit Rev Immunol. 2001;21(1-3):157-78. Crit Rev Immunol. 2001. PMID: 11642602 Review.
Cited by
-
Cross Talk between Cancer and Mesenchymal Stem Cells through Extracellular Vesicles Carrying Nucleic Acids.Front Oncol. 2016 May 23;6:125. doi: 10.3389/fonc.2016.00125. eCollection 2016. Front Oncol. 2016. PMID: 27242964 Free PMC article. Review.
-
Cross-talk between cancer stem cells and immune cells: potential therapeutic targets in the tumor immune microenvironment.Mol Cancer. 2023 Feb 21;22(1):38. doi: 10.1186/s12943-023-01748-4. Mol Cancer. 2023. PMID: 36810098 Free PMC article. Review.
-
Exosomes in the Diagnosis and Treatment of Renal Cell Cancer.Int J Mol Sci. 2023 Sep 20;24(18):14356. doi: 10.3390/ijms241814356. Int J Mol Sci. 2023. PMID: 37762660 Free PMC article. Review.
-
Extracellular vesicles in onco-nephrology.Exp Mol Med. 2019 Mar 15;51(3):1-8. doi: 10.1038/s12276-019-0213-7. Exp Mol Med. 2019. PMID: 30872568 Free PMC article. Review.
-
Biomarker discovery for renal cancer stem cells.J Pathol Clin Res. 2018 Jan 17;4(1):3-18. doi: 10.1002/cjp2.91. eCollection 2018 Jan. J Pathol Clin Res. 2018. PMID: 29416873 Free PMC article. Review.
References
-
- Van Doormaal FF, Kleinjan A, Di Nisio M, Büller HR, Nieuwland R. Cell-derived microvesicles and cancer. Neth J Med. 2009;67:266–273. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

