PSR1 Is a Global Transcriptional Regulator of Phosphorus Deficiency Responses and Carbon Storage Metabolism in Chlamydomonas reinhardtii
- PMID: 26704642
- PMCID: PMC4775146
- DOI: 10.1104/pp.15.01907
PSR1 Is a Global Transcriptional Regulator of Phosphorus Deficiency Responses and Carbon Storage Metabolism in Chlamydomonas reinhardtii
Abstract
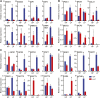
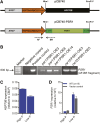
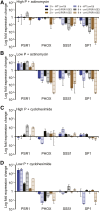
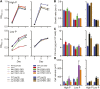
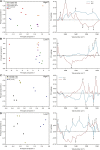
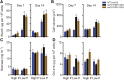
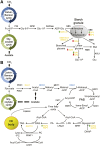
Many eukaryotic microalgae modify their metabolism in response to nutrient stresses such as phosphorus (P) starvation, which substantially induces storage metabolite biosynthesis, but the genetic mechanisms regulating this response are poorly understood. Here, we show that P starvation-induced lipid and starch accumulation is inhibited in a Chlamydomonas reinhardtii mutant lacking the transcription factor Pi Starvation Response1 (PSR1). Transcriptomic analysis identified specific metabolism transcripts that are induced by P starvation but misregulated in the psr1 mutant. These include transcripts for starch and triacylglycerol synthesis but also transcripts for photosynthesis-, redox-, and stress signaling-related proteins. To further examine the role of PSR1 in regulating lipid and starch metabolism, PSR1 complementation lines in the psr1 strain and PSR1 overexpression lines in a cell wall-deficient strain were generated. PSR1 expression in the psr1 lines was shown to be functional due to rescue of the psr1 phenotype. PSR1 overexpression lines exhibited increased starch content and number of starch granules per cell, which correlated with a higher expression of specific starch metabolism genes but reduced neutral lipid content. Furthermore, this phenotype was consistent in the presence and absence of acetate. Together, these results identify a key transcriptional regulator in global metabolism and demonstrate transcriptional engineering in microalgae to modulate starch biosynthesis.
© 2016 American Society of Plant Biologists. All Rights Reserved.
Figures




References
-
- Ball S, Marianne T, Dirick L, Fresnoy M, Delrue B, Decq A (1991) A Chlamydomonas reinhardtii low-starch mutant is defective for 3-phosphoglycerate activation and orthophosphate inhibition of ADP-glucose pyrophosphorylase. Planta 185: 17–26 - PubMed
-
- Ball SG, Morell MK (2003) From bacterial glycogen to starch: understanding the biogenesis of the plant starch granule. Annu Rev Plant Biol 54: 207–233 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

