Further Advances in Optimizing (2-Phenylcyclopropyl)methylamines as Novel Serotonin 2C Agonists: Effects on Hyperlocomotion, Prepulse Inhibition, and Cognition Models
- PMID: 26704965
- PMCID: PMC8317212
- DOI: 10.1021/acs.jmedchem.5b01153
Further Advances in Optimizing (2-Phenylcyclopropyl)methylamines as Novel Serotonin 2C Agonists: Effects on Hyperlocomotion, Prepulse Inhibition, and Cognition Models
Abstract
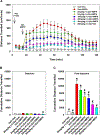
A series of novel compounds with two halogen substituents have been designed and synthesized to further optimize the 2-phenylcyclopropylmethylamine scaffold in the quest for drug-like 5-HT2C agonists. Compound (+)-22a was identified as a potent 5-HT2C receptor agonist, with good selectivity against the 5-HT2B and the 5-HT2A receptors. ADMET assays showed that compound (+)-22a possessed desirable properties in terms of its microsomal stability, and CYP and hERG inhibition, along with an excellent brain penetration profile. Evaluation of (+)-22a in animal models of schizophrenia-related behaviors revealed that it had a desirable activity profile, as it reduced d-amphetamine-stimulated hyperlocomotion in the open field test, it restored d-amphetamine-disrupted prepulse inhibition, it induced cognitive improvements in the novel object recognition memory test in NR1-KD animals, and it produced very little catalepsy relative to haloperidol. These data support the further development of (+)-22a as a drug candidate for the treatment of schizophrenia.
Figures






References
-
- Gray JA; Roth BL The pipeline and future of drug development in schizophrenia. Molecular Psychiatry 2007, 12, 904–922. - PubMed
-
- Harvey PD; Keefe RSE Studies of cognitive change in patients with schizophrenia following novel antipsychotic treatment. Am J Psychiatry 2001, 158, 176–184. - PubMed
-
- Goldberg TE; Goldman RS; Burdick KE; Malhotra AK; Lencz T; Patel RC; Woerner MG; Schooler NR; Kane JM; Robinson DG Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia - Is it a practice effect? Arch Gen Psychiatry 2007, 64, 1115–1122. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

