A pilot study of cetuximab and the hedgehog inhibitor IPI-926 in recurrent/metastatic head and neck squamous cell carcinoma
- PMID: 26705064
- PMCID: PMC5676309
- DOI: 10.1016/j.oraloncology.2015.11.014
A pilot study of cetuximab and the hedgehog inhibitor IPI-926 in recurrent/metastatic head and neck squamous cell carcinoma
Abstract
Background: This phase 1, dose-finding study determined the safety, maximum tolerated dose (MTD)/recommended phase 2 dose (RP2D), antitumor activity, and molecular correlates of IPI-926, a Hedgehog pathway (HhP) inhibitor, combined with cetuximab in patients with relapsed/metastatic squamous cell carcinoma of the head and neck.
Patients and methods: Cetuximab was given with a 400mg/m(2) loading dose followed by 250mg/m(2) weekly. IPI-926 was given daily starting two weeks after cetuximab initiation. A "3+3" study design was used. Prior therapy with cetuximab was allowed. Tumor biopsies occurred prior to cetuximab initiation, prior to IPI-926 initiation, and after treatment with both drugs.
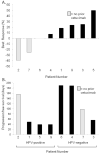
Results: Nine patients were enrolled. The RP2D was 160mg, the same as the single-agent IPI-926 MTD. Among 9 treated, 8 evaluable patients, the best responses were 1 partial response (12.5%), 4 stable disease (50%), and 3 disease progressions (37.5%). The median progression free survival was 77days (95% confidence interval 39-156). Decreases in tumor size were seen in both cetuximab-naïve patients (one HPV-positive, one HPV-negative). The most frequent treatment-emergent adverse events were fatigue, muscle cramps, and rash. No DLTs were observed. Tumor shrinkage and progression free survival were associated with intra-tumoral ErbB and HhP gene expression down-regulation during therapy, supporting the preclinical hypothesis.
Conclusion: Treatment with IPI-926 and cetuximab yielded expected toxicities with signs of anti-tumor activity. Serial tumor biopsies were feasible and revealed proof-of-concept biomarkers.
Keywords: Cetuximab; Combination therapy; Head and neck squamous cell carcinoma; Hedgehog signaling pathway; Phase 1.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
A. Jimeno has received laboratory research support from Infinity. No other authors report conflicts of interest.
Figures


Similar articles
-
A multicenter phase 1 study of PX-866 and cetuximab in patients with metastatic colorectal carcinoma or recurrent/metastatic squamous cell carcinoma of the head and neck.Invest New Drugs. 2014 Dec;32(6):1197-203. doi: 10.1007/s10637-014-0124-3. Epub 2014 Jun 12. Invest New Drugs. 2014. PMID: 24916771 Clinical Trial.
-
Phase II clinical study of valproic acid plus cisplatin and cetuximab in recurrent and/or metastatic squamous cell carcinoma of Head and Neck-V-CHANCE trial.BMC Cancer. 2016 Nov 25;16(1):918. doi: 10.1186/s12885-016-2957-y. BMC Cancer. 2016. PMID: 27884140 Free PMC article. Clinical Trial.
-
Phase I trial of palbociclib, a selective cyclin dependent kinase 4/6 inhibitor, in combination with cetuximab in patients with recurrent/metastatic head and neck squamous cell carcinoma.Oral Oncol. 2016 Jul;58:41-8. doi: 10.1016/j.oraloncology.2016.05.011. Epub 2016 May 28. Oral Oncol. 2016. PMID: 27311401 Free PMC article.
-
Afatinib in squamous cell carcinoma of the head and neck.Expert Opin Pharmacother. 2016 Jun;17(9):1295-301. doi: 10.1080/14656566.2016.1183647. Epub 2016 May 19. Expert Opin Pharmacother. 2016. PMID: 27160335 Review.
-
Toxicities Following Stereotactic Ablative Radiotherapy Treatment of Locally-Recurrent and Previously Irradiated Head and Neck Squamous Cell Carcinoma.Semin Radiat Oncol. 2016 Apr;26(2):112-9. doi: 10.1016/j.semradonc.2015.11.007. Epub 2015 Nov 27. Semin Radiat Oncol. 2016. PMID: 27000507 Review.
Cited by
-
Oncofetal reprogramming in tumor development and progression: novel insights into cancer therapy.MedComm (2020). 2023 Dec 2;4(6):e427. doi: 10.1002/mco2.427. eCollection 2023 Dec. MedComm (2020). 2023. PMID: 38045829 Free PMC article. Review.
-
Radiotherapy plus EGFR inhibitors: synergistic modalities.Cancers Head Neck. 2017 Jan 18;2:2. doi: 10.1186/s41199-016-0020-y. eCollection 2017. Cancers Head Neck. 2017. PMID: 31093349 Free PMC article. Review.
-
Hedgehog signaling in tissue homeostasis, cancers, and targeted therapies.Signal Transduct Target Ther. 2023 Aug 18;8(1):315. doi: 10.1038/s41392-023-01559-5. Signal Transduct Target Ther. 2023. PMID: 37596267 Free PMC article. Review.
-
The use of hedgehog antagonists in cancer therapy: a comparison of clinical outcomes and gene expression analyses.Cancer Biol Ther. 2020 Oct 2;21(10):873-883. doi: 10.1080/15384047.2020.1806640. Epub 2020 Sep 11. Cancer Biol Ther. 2020. PMID: 32914706 Free PMC article.
-
Identification, Culture and Targeting of Cancer Stem Cells.Life (Basel). 2022 Jan 27;12(2):184. doi: 10.3390/life12020184. Life (Basel). 2022. PMID: 35207472 Free PMC article. Review.
References
-
- Burtness B, Bauman JE, Galloway T. Novel targets in HPV-negative head and neck cancer: overcoming resistance to EGFR inhibition. Lancet Oncol. 2013;14:e302–309. - PubMed
-
- Prince MEP, Ailles LE. Cancer Stem Cells in Head and Neck Squamous Cell Cancer. Journal of Clinical Oncology. 2008;26:2871–2875. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

