Antibody-Mediated Rejection in Sensitized Nonhuman Primates: Modeling Human Biology
- PMID: 26705099
- PMCID: PMC4874845
- DOI: 10.1111/ajt.13688
Antibody-Mediated Rejection in Sensitized Nonhuman Primates: Modeling Human Biology
Abstract
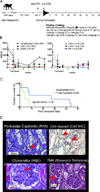
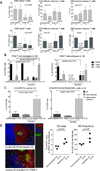
We have established a model of sensitization in nonhuman primates and tested two immunosuppressive regimens. Animals underwent fully mismatched skin transplantation, and donor-specific antibody (DSA) response was monitored by flow cross-match. Sensitized animals subsequently underwent kidney transplantation from their skin donor. Immunosuppression included tacrolimus, mycophenolate, and methylprednisolone. Three animals received basiliximab induction; compared with nonsensitized animals, they showed a shorter mean survival time (4.7 ± 3.1 vs. 187 ± 88 days). Six animals were treated with T cell depletion (anti-CD4/CD8 mAbs), which prolonged survival (mean survival time 21.6 ± 19.0 days). All presensitized animals showed antibody-mediated rejection (AMR). In two of three basiliximab-injected animals, cellular rejection (ACR) was prominent. After T cell depletion, three of six monkeys experienced early acute rejection within 8 days with histological evidence of thrombotic microangiopathy and AMR. The remaining three monkeys survived 27-44 days, with mixed AMR and ACR. Most T cell-depleted animals experienced a rebound of DSA that correlated with deteriorating kidney function. We also found an increase in proliferating memory B cells (CD20(+) CD27(+) IgD(-) Ki67(+) ), lymph node follicular helper T cells (ICOS(+) PD-1(hi) CXCR5(+) CD4(+) ), and germinal center (GC) response. Depletion controlled cell-mediated rejection in sensitized nonhuman primates better than basiliximab, yet grafts were rejected with concomitant DSA rise. This model provides an opportunity to test novel desensitization strategies.
© Copyright 2015 The American Society of Transplantation and the American Society of Transplant Surgeons.
Conflict of interest statement
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Figures





References
-
- Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, Schnitzler MA, et al. OPTN/SRTR 2012 Annual Data Report: Kidney. Am J Transplant. 2013;14(S1):1–44. - PubMed
-
- Eurotransplant International Foundation; 2014. May 17, ANNUAL REPORT 2013. http://www.eurotransplant.org: Report No.
-
- Vo AA, Sinha A, Haas M, Choi J, Mirocha J, Kahwaji J, et al. Factors Predicting Risk for Antibody-mediated Rejection and Graft Loss in Highly Human Leukocyte Antigen Sensitized Patients Transplanted After Desensitization. Transplantation. 2015;99(7):1423–1430. - PubMed
-
- Cecka JM, Kucheryavaya AY, Reinsmoen NL, Leffell MS. Calculated PRA: initial results show benefits for sensitized patients and a reduction in positive crossmatches. Am J Transplant. 2011;11(4):719–724. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

